Nanopatterning
Research on nanopatterning of surfaces by means of self-assembly of block co-polymers.
In the UU groups we have developed methods to make optical meta-materials by self-assembly methods using colloidal templating and thin film deposition techniques. The UU group has developed methods to make inverse opals with independently tunable photonic band gap and optical band gap (Fig. 4), and shown their usefulness in photocatalytic and electrochromic applications.
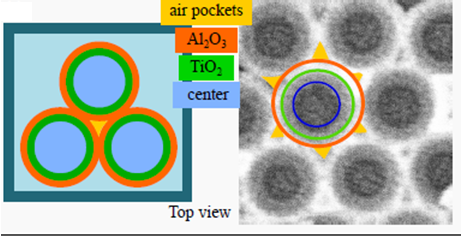
Figure 4: Inverse opal Al2O3 with an inner diameter of 160 nm. A photocatalytic material is either deposited as nanoparticles with high surface area on the inner walls of the Al2O3 scaffold, or as additional layers deposited on the walls, e.g. by atomic layer deposition (ALD), to independently modify the photonic and electronic band gap properties of the inverse opal structure.
In the fields of nanochromics and tunable optical filters, UU performs research on nanopatterning of surfaces by means of self-assembly of block co-polymers. The template can have various shape as function of volume fraction of hydrophobic polymer to achieve nanostructured spherical or cylinder patterns on the substrate. Nanostructured films prepared along these lines can e.g. improve optical transparency when it used as substrate for chromic thin films, or make films with graded refractive index, thin optical thickness and large exposed surface area, and also allowing for making multilayer films with selective electron and phonon transport properties. New directions in the research includes development of new polymer template structures. Fig. 5 illustrates the nanorod template synthesis using block-copolymers for making nanorod structures.
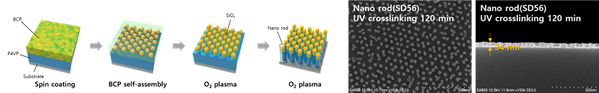
Figure 5: Synthesis of nanorods using the block-copolymer (PS-b-PDMS) /P4VP, where P4VP determines the height of the nanopattern. The dot pattern on P4VP polymer, acts as etching mask to make the rod structure.
After immersion in metal ion solution, it is possible to make metal oxide nanostructure after ICP-RIE processing. Nanotemplating can be used to make multichromic and transparent conducting devices. For example, highly visible light transmittant thermochromic thin film, metal oxide (MO), or OMO, structures using nanostructured thermochromic MO and ITO materials, can be made, thus making multifunctional transparent conducting materials. In particular TCO for optoelectronic and chromogenic applications.
References
[26] S. Kim et al., Transparent conductive oxide thin film sandwich structures exhibiting enhanced optical and thermoelectric properties, Adv. Mater (2022).
[27] Hui-Ying Qu, Jun-Xin Wang, José Montero, and Gunnar A. Niklasson, Lars Österlund, Electrochromic and Structurally Colored Nickel Oxide Films, J. Appl. Phys. 129, 123105 (2021); https://doi.org/10.1063/5.0043673
[28] D. M. Lebrun, PhD Thesis, Uppsala University (2017) http://urn.kb.se/resolve?urn=urn:nbn:se:uu:diva-300408
[29] D. M. Lebrun, and L. Österlund, Demonstration of slow photon chemistry on multilayer inverse opals, Sci. Adv. Mater. 9(11) (2017) 1947-1952. https://doi.org/10.1166/sam.2017.2833
[30] L. Österlund, A. W. Grant, and B. Kasemo, Lithographic Techniques in Nanocatalysis, in Nanocatalysis, Eds: U. Heiz, and U. Landman, Nanocatalysis, Series: NanoScience and Technology, Springer-Verlag Berlin Heidelberg (2007), pp 269-341. ISBN 978-3-540-74551-8. http://www.springer.com/gp/book/9783540326458. (citations: 187).
[31] L. Österlund, S. Kielbassa, C. Werdinius, and B. Kasemo, Reactivity of Pt/ceria and Pt/alumina planar model catalysts prepared by colloidal lithography, J. Catal. 215, 94 (2003).
