Hellman lab
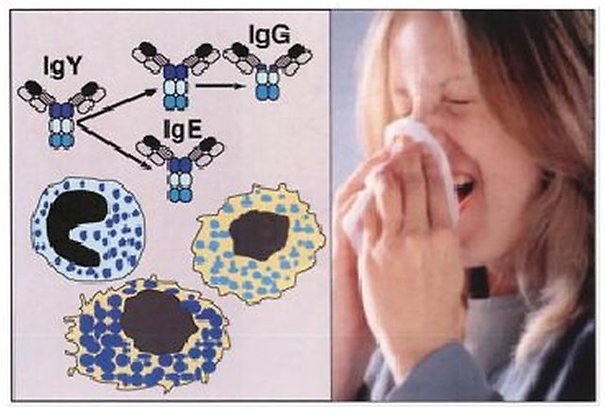
A dramatic increase in the incidence of atopic (IgE mediated) allergies has occurred during the past 50 years. Estimates show that 20-30% of the populations in some western countries have allergic symptoms that are so severe, they constitute a medical problem. This situation has, together with better management of many of the major infectious diseases, placed atopic allergies among the major medical issues of the industrialized world. Common atopic allergies include hay fever, fur allergies, different types of asthma, food allergies and dermatitis.
Popular science presentation
A dramatic increase in the incidence of atopic (IgE mediated) allergies has occurred during the past 50 years. Estimates show that 20-30% of the populations in some western countries have allergic symptoms that are so severe, they constitute a medical problem. This situation has, together with better management of many of the major infectious diseases, placed atopic allergies among the major medical issues of the industrialized world. Common atopic allergies include hay fever, fur allergies, different types of asthma, food allergies and dermatitis.
Despite its normally very low concentration in human plasma, immunoglobulin IgE is the central player in these allergies. IgE triggers the allergic reaction through its interaction with the high affinity receptor for IgE on mast cells and basophils. These two related cell types have the potential to induce strong inflammatory reactions by releasing a number of preformed substances, such as histamine, heparin, cytokines and proteases. During activation mast cells and basophils also produce potent physiologically acting lipid mediators, i.e. prostaglandins and leukotrienes. In addition to playing a pivotal role in allergy and asthma, these two cell types play an important role in our defense against bacterial and parasite infections and in other diseases like arthritis and cancer.
In order to obtain a better picture of the basic regulatory mechanisms of atopic allergies and thereby to find new ways to interfere with these processes, we study different aspects of cells and molecules involved in these diseases. Our main areas of research are IgE structure and evolution, mast cell and basophil biology and the development of novel treatment strategies. The treatment strategies we are exploring are vaccinations against several key cytokines that work as master regulators of TH2 mediated inflammatory conditions, i.e. IL-18, IL-33 and thymic stromal lymphopoietin (TSLP).
Group members
Publications
Part of Cells, 2024
- DOI for Cultures of Human Skin Mast Cells, an Attractive In Vitro Model for Studies of Human Mast Cell Biology
- Download full text (pdf) of Cultures of Human Skin Mast Cells, an Attractive In Vitro Model for Studies of Human Mast Cell Biology
Part of International Journal of Molecular Sciences, 2024
- DOI for Extended Cleavage Specificity of two Hematopoietic Serine Proteases from a Ray-Finned Fish, the Spotted Gar (Lepisosteus oculatus)
- Download full text (pdf) of Extended Cleavage Specificity of two Hematopoietic Serine Proteases from a Ray-Finned Fish, the Spotted Gar (Lepisosteus oculatus)
Part of International Journal of Molecular Sciences, 2024
- DOI for The Extended Cleavage Specificity of Channel Catfish Granzyme-like II, A Highly Specific Elastase, Expressed by Natural Killer-like Cells
- Download full text (pdf) of The Extended Cleavage Specificity of Channel Catfish Granzyme-like II, A Highly Specific Elastase, Expressed by Natural Killer-like Cells
Part of Frontiers in Immunology, 2023
- DOI for Extended cleavage specificities of human granzymes A and K, two closely related enzymes with conserved but still poorly defined functions in T and NK cell-mediated immunity
- Download full text (pdf) of Extended cleavage specificities of human granzymes A and K, two closely related enzymes with conserved but still poorly defined functions in T and NK cell-mediated immunity
Part of Journal of Immunology Research, 2023
- DOI for House Dust Mite and Cat Dander Extract Induce Asthma-Like Histopathology with an Increase of Mucosal Mast Cells in a Guinea Pig Model
- Download full text (pdf) of House Dust Mite and Cat Dander Extract Induce Asthma-Like Histopathology with an Increase of Mucosal Mast Cells in a Guinea Pig Model
Identification of the Major Protein Components of Human and Cow Saliva
Part of International Journal of Molecular Sciences, 2023
- DOI for Identification of the Major Protein Components of Human and Cow Saliva
- Download full text (pdf) of Identification of the Major Protein Components of Human and Cow Saliva
Phenotypic and Functional Heterogeneity of Monocytes and Macrophages
Part of International Journal of Molecular Sciences, 2023
- DOI for Phenotypic and Functional Heterogeneity of Monocytes and Macrophages
- Download full text (pdf) of Phenotypic and Functional Heterogeneity of Monocytes and Macrophages
Part of Scientific Reports, p. 16261, 2023
- DOI for Single-cell transcriptomics delineates the immune cell landscape in equine lower airways and reveals upregulation of FKBP5 in horses with asthma.
- Download full text (pdf) of Single-cell transcriptomics delineates the immune cell landscape in equine lower airways and reveals upregulation of FKBP5 in horses with asthma.
Part of Developmental and Comparative Immunology, 2023
- DOI for Two granzyme A/K homologs in Zebra mbuna have different specificities, one classical tryptase and one with chymase activity
- Download full text (pdf) of Two granzyme A/K homologs in Zebra mbuna have different specificities, one classical tryptase and one with chymase activity
Part of Developmental and Comparative Immunology, 2022
- DOI for Analysis of the mast cell expressed carboxypeptidase A3 and its structural and evolutionary relationship to other vertebrate carboxypeptidases
- Download full text (pdf) of Analysis of the mast cell expressed carboxypeptidase A3 and its structural and evolutionary relationship to other vertebrate carboxypeptidases
Part of Developmental and Comparative Immunology, 2022
- DOI for Chicken cathepsin G-like - A highly specific serine protease with a peculiar tryptase specificity expressed by chicken thrombocytes
- Download full text (pdf) of Chicken cathepsin G-like - A highly specific serine protease with a peculiar tryptase specificity expressed by chicken thrombocytes
Part of Developmental and Comparative Immunology, 2022
- DOI for Extended cleavage specificity of a Chinese alligator granzyme B homologue, a strict Glu-ase in contrast to the mammalian Asp-ases
- Download full text (pdf) of Extended cleavage specificity of a Chinese alligator granzyme B homologue, a strict Glu-ase in contrast to the mammalian Asp-ases
Part of Cancers, 2022
- DOI for Platelet-Derived PDGFB Promotes Recruitment of Cancer-Associated Fibroblasts, Deposition of Extracellular Matrix and Tgf beta Signaling in the Tumor Microenvironment
- Download full text (pdf) of Platelet-Derived PDGFB Promotes Recruitment of Cancer-Associated Fibroblasts, Deposition of Extracellular Matrix and Tgf beta Signaling in the Tumor Microenvironment
Part of International Journal of Molecular Sciences, 2022
- DOI for Quantitative Analysis of the Transcriptome of Two Commonly Used Human Monocytic Cell Lines-THP-1 and Mono Mac 6-Reveals Their Arrest during Early Monocyte/Neutrophil Differentiation
- Download full text (pdf) of Quantitative Analysis of the Transcriptome of Two Commonly Used Human Monocytic Cell Lines-THP-1 and Mono Mac 6-Reveals Their Arrest during Early Monocyte/Neutrophil Differentiation
Part of International Journal of Molecular Sciences, 2022
- DOI for Quantitative In-Depth Transcriptome Analysis Implicates Peritoneal Macrophages as Important Players in the Complement and Coagulation Systems
- Download full text (pdf) of Quantitative In-Depth Transcriptome Analysis Implicates Peritoneal Macrophages as Important Players in the Complement and Coagulation Systems
Part of International Journal of Molecular Sciences, 2022
- DOI for Quantitative Transcriptome Analysis of Purified Equine Mast Cells Identifies a Dominant Mucosal Mast Cell Population with Possible Inflammatory Functions in Airways of Asthmatic Horses
- Download full text (pdf) of Quantitative Transcriptome Analysis of Purified Equine Mast Cells Identifies a Dominant Mucosal Mast Cell Population with Possible Inflammatory Functions in Airways of Asthmatic Horses
The Human Monocyte: A Circulating Sensor of Infection and a Potent and Rapid Inducer of Inflammation
Part of International Journal of Molecular Sciences, 2022
- DOI for The Human Monocyte: A Circulating Sensor of Infection and a Potent and Rapid Inducer of Inflammation
- Download full text (pdf) of The Human Monocyte: A Circulating Sensor of Infection and a Potent and Rapid Inducer of Inflammation
Part of Cancer Immunology and Immunotherapy, p. 2029-2040, 2022
- DOI for Vaccination against galectin-1 promotes cytotoxic T-cell infiltration in melanoma and reduces tumor burden
- Download full text (pdf) of Vaccination against galectin-1 promotes cytotoxic T-cell infiltration in melanoma and reduces tumor burden
Part of PLOS ONE, 2021
- DOI for Duodenases are a small subfamily of ruminant intestinal serine proteases that have undergone a remarkable diversification in cleavage specificity
- Download full text (pdf) of Duodenases are a small subfamily of ruminant intestinal serine proteases that have undergone a remarkable diversification in cleavage specificity
Part of Biological chemistry (Print), p. 861-867, 2021
- DOI for Marked difference in efficiency of the digestive enzymes pepsin, trypsin, chymotrypsin, and pancreatic elastase to cleave tightly folded proteins
- Download full text (pdf) of Marked difference in efficiency of the digestive enzymes pepsin, trypsin, chymotrypsin, and pancreatic elastase to cleave tightly folded proteins
Part of International Journal of Molecular Sciences, 2021
- DOI for Mast Cells and Basophils in the Defense against Ectoparasites: Efficient Degradation of Parasite Anticoagulants by the Connective Tissue Mast Cell Chymases
- Download full text (pdf) of Mast Cells and Basophils in the Defense against Ectoparasites: Efficient Degradation of Parasite Anticoagulants by the Connective Tissue Mast Cell Chymases
Part of International Journal of Molecular Sciences, 2020
- DOI for Extended Cleavage Specificities of Two Mast Cell Chymase-Related Proteases and One Granzyme B-Like Protease from the Platypus, a Monotreme
- Download full text (pdf) of Extended Cleavage Specificities of Two Mast Cell Chymase-Related Proteases and One Granzyme B-Like Protease from the Platypus, a Monotreme
Part of International Journal of Molecular Sciences, 2020
- DOI for Extended Cleavage Specificity of the Rat Vascular Chymase, a Potential Blood Pressure Regulating Enzyme Expressed by Rat Vascular Smooth Muscle Cells
- Download full text (pdf) of Extended Cleavage Specificity of the Rat Vascular Chymase, a Potential Blood Pressure Regulating Enzyme Expressed by Rat Vascular Smooth Muscle Cells
Part of Cells, 2020
- DOI for How Relevant Are Bone Marrow-Derived Mast Cells (BMMCs) as Models for Tissue Mast Cells?: A Comparative Transcriptome Analysis of BMMCs and Peritoneal Mast Cells
- Download full text (pdf) of How Relevant Are Bone Marrow-Derived Mast Cells (BMMCs) as Models for Tissue Mast Cells?: A Comparative Transcriptome Analysis of BMMCs and Peritoneal Mast Cells
Part of Frontiers in Immunology, 2020
- DOI for Mouse Strain-Dependent Difference Toward the Staphylococcus aureus Allergen Serine Protease-Like Protein D Reveals a Novel Regulator of IL-33
- Download full text (pdf) of Mouse Strain-Dependent Difference Toward the Staphylococcus aureus Allergen Serine Protease-Like Protein D Reveals a Novel Regulator of IL-33
Part of Allergy. European Journal of Allergy and Clinical Immunology, p. 707-708, 2020
- DOI for Novel aspects of mast cell and basophil function: Highlights from the 9th meeting of the European Mast Cell and Basophil Research Network (EMBRN)-A Marcus Wallenberg Symposium
- Download full text (pdf) of Novel aspects of mast cell and basophil function: Highlights from the 9th meeting of the European Mast Cell and Basophil Research Network (EMBRN)-A Marcus Wallenberg Symposium
Part of International Journal of Molecular Sciences, 2020
- DOI for Potent and Broad but not Unselective Cleavage of Cytokines and Chemokines by Human Neutrophil Elastase and Proteinase 3
- Download full text (pdf) of Potent and Broad but not Unselective Cleavage of Cytokines and Chemokines by Human Neutrophil Elastase and Proteinase 3
Protective role of mouse mast cell tryptase Mcpt6 in melanoma.
Part of Pigment Cell & Melanoma Research, p. 579-590, 2020
Part of CELLS, 2020
- DOI for Quantitative In-Depth Analysis of the Mouse Mast Cell Transcriptome Reveals Organ-Specific Mast Cell Heterogeneity
- Download full text (pdf) of Quantitative In-Depth Analysis of the Mouse Mast Cell Transcriptome Reveals Organ-Specific Mast Cell Heterogeneity
Part of Molecular and biochemical parasitology (Print), p. 29-38, 2019
Part of International Journal of Molecular Sciences, 2019
- DOI for Extended Cleavage Specificities of Rabbit and Guinea Pig Mast Cell Chymases: Two Highly Specific Leu-Ases
- Download full text (pdf) of Extended Cleavage Specificities of Rabbit and Guinea Pig Mast Cell Chymases: Two Highly Specific Leu-Ases
Part of Developmental and Comparative Immunology, p. 160-169, 2019
Part of International Journal of Molecular Sciences, 2019
- DOI for Highly Selective Cleavage of TH2-Promoting Cytokines by the Human and the Mouse Mast Cell Tryptases, Indicating a Potent Negative Feedback Loop on TH2 Immunity
- Download full text (pdf) of Highly Selective Cleavage of TH2-Promoting Cytokines by the Human and the Mouse Mast Cell Tryptases, Indicating a Potent Negative Feedback Loop on TH2 Immunity
Part of PLOS ONE, 2018
- DOI for Extended cleavage specificities of mast cell proteases 1 and 2 from golden hamster: Classical chymase and an elastolytic protease comparable to rat and mouse MCP-5
- Download full text (pdf) of Extended cleavage specificities of mast cell proteases 1 and 2 from golden hamster: Classical chymase and an elastolytic protease comparable to rat and mouse MCP-5
Part of PLOS ONE, 2018
Part of Frontiers in Immunology, 2018
- DOI for Extended Cleavage Specificity of Human Neutrophil Elastase, Human Proteinase 3, and Their Distant Ortholog Clawed Frog PR3-Three Elastases With Similar Primary but Different Extended Specificities and Stability
- Download full text (pdf) of Extended Cleavage Specificity of Human Neutrophil Elastase, Human Proteinase 3, and Their Distant Ortholog Clawed Frog PR3-Three Elastases With Similar Primary but Different Extended Specificities and Stability
Part of Virulence, p. 879-894, 2018
- DOI for Secreted Giardia intestinalis cysteine proteases disrupt intestinal epithelial cell junctional complexes and degrade chemokines
- Download full text (pdf) of Secreted Giardia intestinalis cysteine proteases disrupt intestinal epithelial cell junctional complexes and degrade chemokines
Part of The FASEB Journal, p. 1204-1214, 2017
Part of Journal of Immunology, p. 1474-1483, 2017
Part of PLOS ONE, 2016
- DOI for Asp-ase Activity of the Opossum Granzyme B Supports the Role of Granzyme B as Part of Anti-Viral Immunity Already during Early Mammalian Evolution
- Download full text (pdf) of Asp-ase Activity of the Opossum Granzyme B Supports the Role of Granzyme B as Part of Anti-Viral Immunity Already during Early Mammalian Evolution
Part of Developmental And Comparative Immunology, p. 84-95, 2016
Elimination Of Il-33 Inhibits Airways Hyperresponsiveness
Part of American Journal of Respiratory and Critical Care Medicine, 2016
Identification of Biological and Pharmaceutical Mast Cell- and Basophil-Related Targets
Part of Scandinavian Journal of Immunology, p. 465-472, 2016
Part of PLOS ONE, 2015
- DOI for Granule Associated Serine Proteases of Hematopoietic Cells - An Analysis of Their Appearance and Diversification during Vertebrate Evolution
- Download full text (pdf) of Granule Associated Serine Proteases of Hematopoietic Cells - An Analysis of Their Appearance and Diversification during Vertebrate Evolution
IgA deficiency in wolves from Canada and Scandinavia
Part of Developmental and Comparative Immunology, p. 26-28, 2015
Part of PLOS ONE, 2015
- DOI for rMCP-2, the Major Rat Mucosal Mast Cell Protease, an Analysis of Its Extended Cleavage Specificity and Its Potential Role in Regulating Intestinal Permeability by the Cleavage of Cell Adhesion and Junction Proteins
- Download full text (pdf) of rMCP-2, the Major Rat Mucosal Mast Cell Protease, an Analysis of Its Extended Cleavage Specificity and Its Potential Role in Regulating Intestinal Permeability by the Cleavage of Cell Adhesion and Junction Proteins
The Importance of Exosite Interactions for Substrate Cleavage by Human Thrombin
Part of PLOS ONE, 2015
- DOI for The Importance of Exosite Interactions for Substrate Cleavage by Human Thrombin
- Download full text (pdf) of The Importance of Exosite Interactions for Substrate Cleavage by Human Thrombin
Part of PLOS ONE, 2015
- DOI for Vaccination against IL-33 Inhibits Airway Hyperresponsiveness and Inflammation in a House Dust Mite Model of Asthma
- Download full text (pdf) of Vaccination against IL-33 Inhibits Airway Hyperresponsiveness and Inflammation in a House Dust Mite Model of Asthma
Characterization of an Experimental Vaccine for Bovine Respiratory Syncytial Virus
Part of Clinical and Vaccine Immunology, p. 997-1004, 2014
Fc Receptors for Immunoglobulins and Their Appearance during Vertebrate Evolution
Part of PLOS ONE, 2014
- DOI for Fc Receptors for Immunoglobulins and Their Appearance during Vertebrate Evolution
- Download full text (pdf) of Fc Receptors for Immunoglobulins and Their Appearance during Vertebrate Evolution
Hellman lab
Research projects
1. Mast cell and Basophil Biology
Mammalian mast cells and basophilic leukocytes; studies of their phenotype, function and development.
A number of cell type specific differentiation markers for human and rodent mast cells and basophilic leukocytes have been isolated by cloning of their corresponding cDNAs or genes. These markers are presently being used to study mast cell and basophil differentiation and in vivo distribution. Recombinant proteases are being produced for studies of cleavage specificity and function.
Granule stored serine proteases of mast cells, basophils, NK cells, T cells and neutrophils.
Serine proteases are major constituents of the cytoplasmic granules in mast cells, neutrophils, T cells and NK cells. These proteases can account for up to 50% of the total cellular protein and they are stored in their active form in the cell (19, 36). The genes encoding the granule-stored proteases are arranged in four different loci, the mast cell chymase locus, the mast cell tryptase locus, the met-ase locus and the T cell tryptase locus. In almost all placental mammals investigated, the mast cell chymase locus holds at least one mast cell expressed enzyme; the α-chymase, one neutrophil protease; cathepsin G and two T cell and NK cell expressed genes: the granzymes B and H. The mast cell tryptase locus contains the mast cell tryptases and also membrane bound tryptases also expressed by other cell types (102). The met-ase locus contains granzyme M and several neutrophil proteases, i.e. N-elastase, proteinase 3 and the antibacterial but proteolytically inactive azurocidin. This locus also harbors the complement factor D (adipsin) and another serine protease of yet unknown function, Prss L1, that is expressed in islet cells of the pancreas. The T cell tryptase locus encodes granzymes A and K.
The T cell granzymes have been shown to be responsible for caspase dependent and caspase independent activation of apoptosis in target cells. The neutrophil proteases are involved in paving the way for these cells during their migration to inflamed tissue and several of them also have anti-bacterial properties. The mast cell chymase and the tryptases have been shown to cleave a number of substrates, in vitro. However their in vivo functions are less well understood.
In several mammalian species the chymase locus has expanded by numerous gene duplication events. In mice, instead of the 4 genes found in the human locus, there are 15 functional chymase locus genes and in rats there are 28 (84, 86). In rodents, two new subfamilies are also among these duplicated genes, the αchymases and the mouse mast cell protease (mMCP)-8 family. mMCP-8 was cloned by our group a number of years ago. This gene has been found to be expressed only by basophils and was the first cloned basophil specific marker in any species (40, 50, 55, 90, 97). The mMCP-8 gene is presently used as a marker for basophil development and also to construct basophil depleted animals.
One of our main areas of research is related to the emergence of these enzymes during vertebrate evolution (36, 53, 83). A more detailed view of their appearance can hopefully help us find the central evolutionary conserved function of these enzymes and also see if divergent, convergent or both processes have participated in their emergence and diversification. Both the chymase and the tryptases are presently evaluated as potential targets for the treatment of allergies and other mast cell dependent inflammatory disorders, which increase the interest for these enzymes from a medical point of view.
In addition to their tissue location and evolution, the most important characteristic of these proteases is their cleavage specificity and their in vivo targets. To obtain a detailed picture of their extended specificity we use a screening system involving a library of more than 50 million random nonamers presented on the surface of T7 phages. This phage display system makes it possible to obtain a detailed picture of the cleavage specificity involving up to nine amino acids surrounding the cleavage site. To be able to verify the result and to obtain kinetic data on the cleavage reaction we have recently developed a system of recombinant substrates. With these novel substrates we have been able to verify the result from the phage display and also get detailed information on the influence of individual amino acid substitutions on the cleavage rate.
Using these recombinant substrates we have recently determined the cleavage specificity of a number of different mast cell enzymes and also performed a detailed study of human thrombin. The thrombin study turned out to be very interesting as it showed that many natural substrates often are relatively poor substrates for this enzyme and are most likely highly dependent on exosite interactions to be actively cleaved in vivo (108).
The projects related to the various hematopoietic serine proteases are listed here below.
A. Evolution of gene loci
We have performed a detailed analysis on the structure of the chymase locus in mammals, including a panel of placental mammals, one marsupial, the American opossum and on one monotreme, the Australian platypus (84, 86). The results show that massive gene duplications have expanded this locus in several mammalian lineages. The most extensive expansion has occurred in rodents. Interestingly, independent duplications have resulted in a relatively similar expansion in rats and mice during the past 15-20 million years, indicating that environmental factors have been a strong force in this expansion. Dramatic changes and the appearance of new enzymes, the duodenases, have also occurred in ruminats (Fig 1)(86). Interestingly, the dueodenases have changed their tissue specificity and function; from originally being immune proteases they are now expressed in the intestinal region where they participate in food digestion. We then performed an analysis of the mast cell tryptase locus that showed that this locus has been relatively well preserved during the past 140-200 million years of mammalian evolution (102). Distantly related enzymes, both to the chymase and the tryptase locus genes, are also found in fish, however their tissue location and cleavage specificity is not yet known (83).
The ongoing project is presently focusing on the cleavage specificity of a few proteases from the chymase locus of the platypus, several chymases from a diverse set of placental mammals, the human neutrophil proteases, N-elastase, cathepsin G and proteinase 3, the human T cell granzymes A and K, the opossum granzyme B and several fish proteases distantly related to the chymase locus genes of mammals. We are also focusing on completing an evolutionary analysis of the T cell granzyme and the granzyme M loci.
B. Cleavage specificity
A major effort during the last 10 years has been the characterization of the extended specificity of a selected panel of hematopoietic serine proteases (63, 71, 91, 92, 95, 96, 101, 103). One of the first enzymes studied was the rat α-chymase (rMCP-5). This protease was found to have changed primary specificity, from a chymase to an elastase (71). The subsequent question was if rodents have a counterpart of the human mast cell chymase. Mice and rats have several additional closely related genes, the βchymases (Fig 1B). Analysis of one of the major mouse βchymases, mMCP-4, did indeed show that this protease has a cleavage specificity that is almost identical to the human chymase (91). The chymase specificity was later shown to have been a key feature of mast cells during more than 140 million years of mammalian evolution. By analysis of the mast cell chymase from the American opossum we were able to show that this enzyme is also a chymase with very similar specificity as the human chymase (92).
C. General mast cell and basophil biology
The granule-associated serine proteases are stored in an active form tightly bound to negatively charged polysaccharides. In mast cells and basophils, these carbohydrates are either present as heavily sulfated heparin or chondroitin sulfate. These carbohydrates are synthesized on a protein core, the serglycin core protein. The mouse serglycin cDNA and gene were cloned by us a number of years ago (17, 18). Interestingly, knocking out this core protein results in problems for the cell to store theses granule associated proteases (89). Knocking out one of the enzymes involved in the synthesis of heparin, NDST-2, also results in mast cells lacking heparin and cells that have difficulty in storing several of the proteases in their granula (47). The granule-associated proteases can also be used as markers to study mast cell differentiation and to characterize mast cell lines. Using these criteria we have identified a mouse cell line with both mast cell and basophil characteristics indicating an early bipotential precursor (52). In addition, these criteria have been used to study the kinetics of mast cell expansion during various parasite infections (43, 50) and the phenotype of a number of human mast cell, basophil and monocytic cell lines (21, 24, 25, 27, 28, 34, 35). A number of years ago we developed antisera against most of the different proteases stored in various mouse mast cell populations. These antisera have been used by a number of labs all over the world to study mast cell function and differentiation (47, 51, 57, 62, 89).
We have a long-term interest in basophil biology, which has resulted in one publication on establishing culture conditions and purification scheme for immature human basophils. This project was initiated to obtain sufficient amounts of transcriptionally active basophil precursors to study the transcriptome of human basophils (85). Mature blood basophils are mostly terminally differentiated and lack almost entirely functional mRNA (85). An additional manuscript is presently being completed on the analysis of the transcriptome of these in vitro differentiated human basophil precursors (manuscript in preparation).
2. IgE –Evolution and Homeostasis
Structure, function and evolution of IgE and IgE-like immunoglobulin isotypes.
To study the evolution of IgE and IgG like isotypes, four different isotypes has been isolated from the american opossum, a marsupial, and five isotypes from the australian platypus (an egg laying mammal). Our results show that modern IgG and IgE appeared already very early during mammalian evolution, possibly more than 250-300 million years ago.
Diversity in constant and variable regions of the immunoglobulin heavy and light chain loci in non-placental mammals.
To study the adaptive immune system and its increase in complexity during vertebrate evolution, we have turned our interest to mammals distantly related to placental mammals, marsupials and monotremes. There are only three extant species of monotremes that exist; the duck-billed platypus and two species of echidna. We have focused our efforts on the appearance of the various immunoglobulin isotypes, with specific emphasis on IgE, and on the diversification of the (variable) V gene pool in the heavy and light chains. However, we have also participated in the analysis of T-cell receptor and MHC repertoire in non-placental mammals (67, 68, 72, 77, 82).
Our aim has been to study the variability in the antibody repertoire and how it has developed during vertebrate evolution. The result from a screening and analysis of a large number of clones from a platypus spleen cDNA library showed that the V gene segments of the heavy chain locus in the platypus belong to what appears to be only one V gene family (64). However, this family is highly diversified. In contrast, humans and mice have seven and fifteen highly diversified heavy chain V gene families, respectively. Diversity in the platypus is instead created through a large number of joining (J) gene segments and fairly long, and highly diversified (D) gene segments (64). A similar pattern was also seen in the lambda light chain locus of the platypus (80). Two closely related germ line V gene families account for all variability in the lambda locus. However, this limited number of V gene families is well compensated for by a large variation in the complementarity determining regions (80). Our results indicate that several rounds of gene loss and re-expansions of the remaining V heavy and light chain genes have occurred during vertebrate evolution. These events have been key in shaping the present diversity in the V gene repertoire of various mammalian species (64, 78, 80).
Diversification of receptors interacting with the constant regions of immunoglobulins during the past 450 million years of vertebrate evolution.
The number of immunoglobulin isotypes that are expressed in a given species has increased quite dramatically during the past 450 million years of vertebrate evolution. Various fish species normally express 2 or 3 immunoglobulin isotypes of which not one undergoes an isotype switch. In contrast, placental mammals frequently have 8-9 isotypes or sometimes even more. The majority of the 9 isotypes in humans are also so called post switch isotypes. They are expressed after an isotype switching event, from a B cell originally expressing IgM. These new isotypes give the antigen binding new biological functions. IgA, which is one of these new isotypes, has the capacity to be transferred into saliva, tears and intestinal secretions and can thereby interact with the antigen before it enters our body fluids. IgE can bind to high affinity receptors on mast cells and basophils and thereby trigger the release of histamine and other potent mediators. IgG binds to receptors on phagocytes like macrophages and neutrophils and thereby facilitate efficient clearance of bacteria and various immune complexes.
For several years we have been working on the evolution of the various post switch isotypes, particularly IgG and IgE, and have seen that these isotypes appeared during early mammalian evolution, 220-350 million years ago (42,48, 59, 60, 61, 65, 69, 70, 74, 98, 100). Our interest now focuses on the evolution of receptors interacting with these new isotypes and thereby how these isotypes acquired their characteristic biological functions. Here, one of the more important questions is when adaptive immunity, through immunoglobulins, became regulators of mast cell function by binding to mast cell specific receptors; the high affinity receptor for IgE (FcεRI).
General IgE homeostastsis and the characterization of novel splice variants for IgE in primates.
IgE is present at very low plasma levels in most mammals. The concentration of IgE in non-allergic humans is generally between 10 and 400 ng/ml, whereas IgG levels are 10 000 to 1 million times higher and range from 8-16 mg/ml. To obtain a better understanding of the regulation of IgE we have studied the levels of IgE in domestic and non-domestic animal populations. The levels of IgE in domestic dog populations were found to be 10-100 times higher than what is observed in laboratory mice and humans ranging from 500 ng to 50 µg/ml. The IgE levels in the wild Scandinavian wolf population were found to be twice as high as in the domestic dogs (81, 94) and horses have even higher IgE levels. In general it appears as if IgE levels in wild animal populations with high parasite loads are10-100 times higher than in domestic animals free of parasite worm infections (87, 94).
A few years ago I wrote a review on the regulation of IgE homeostasis (87). This review, which summarizes much of what we know about IgE and its regulation and also lists a number of potential targets for intervention is included as a pdf file.
During an analysis of IgE transcripts from the IgE producing cell line U266, we identified a number of novel splice variants (23). Subsequently these transcripts were not found in rats (31) but appear to be primate specific. Most of the transcripts are probably non-functional, however, one or two of them may have novel roles in the regulation of IgE synthesis (23, 31). One of them is particularly interesting because it indicates that IgE producing B cells may have two separate signaling pathways, which may have implications for allergy development in humans (23).
3. Novel Treatment Strategies
Development of a novel allergy vaccine for the treatment of atopic allergies.
A novel approach for the treatment of atopic allergies is being developed. This is based on the induction of a strong therapeutic anti-IgE response in the host. The vaccine has been found to drastically reduce the allergen sensitivity after 8-10 weeks of treatment.
Generation of therapeutic anti-IgE, anti-IL-18, anti-TSLP and anti-IL-33 antibody responses through vaccination.
The collected information from the various projects described above has boosted our interest to use this information to develop novel treatment strategies against IgE mediated allergies. One of the most rewarding achievements as a scientist in basic research is to take conceptual studies and use that knowledge for treatment in the clinic. To increase our chances to reach the clinic, we have focused our efforts on one major project. We develop therapeutic vaccines targeting key molecules in the allergic cascade. This is to obtain a relatively cost-effective treatment method that also has the potential of being used outside of the western world (Fig 3). Further, the technology developed during this process has also been adopted in collaborative attempts to develop vaccines in other areas including cancer and autoimmunity (104,105).
Despite its normally very low concentration in human plasma, IgE is a major cause of allergies. Based on the assumption that a substantial reduction in total and antigen specific IgE will lead to improved conditions in atopic individuals, we have studied the possibility of reducing the levels of circulating and mast cell bound IgE in the host by vaccination. In the initial studies a fusion protein consisting of the constant domains 2 and 3 (CH2 and CH3) of rat IgE coupled to glutathione-S-transferase (GST) from Schistosoma japonicum was used as active component in the allergy vaccine. Injection of GST-C2C3 together with adjuvant resulted in a potent therapeutic anti-IgE response in a number of rat strains. Vaccination of ovalbumin sensitized Wistar rats resulted in a profound decrease in serum IgE levels as well as a nearly complete block in histamine release upon challenge with either a cross-linking polyclonal anti-IgE serum or an allergen (26). New soluble vaccine components containing only the CH3 domain of IgE (the IgE receptor interacting domain), flanked by the second and fourth constant domains of the opossum IgE heavy chain, was later produced and tested in the rat animal model. Compared to previous vaccines this vaccine was found to be almost 30 times more potent at inducing a therapeutic antibody response (58). In addition, we demonstrated that the response was reversible with time and that no species cross reactivity was observed (58). These results suggested that the vaccine strategy should proceed to toxicity testing and clinical trials in humans.
A company was formed around the concept, Resistentia Pharmaceuticals AB. However,a number of contentious decisions within the company, notably concerning the choice of adjuvant, became apparent. When targeting a self-molecule such as IgE, a strong adjuvant must be used. Despite this knowledge, a decision from the higher powers was made to proceed with the adjuvant alum in clinical trials. In numerous studies within the company, alum had been shown to have almost no enhancing activity on the anti–IgE response. Predictably from the preclinical studies, no or only very minor effects on circulating IgE levels were seen in the clinical trial and the project had to be discontinued due to lack of funding (93). The results from the animal models also indicated that it was difficult to reduce IgE to baseline levels when IgE levels exceeded 200 ng/ml; a prerequisite for good clinical effect (93). The patient group that most urgently need new treatment methods, including many patients with severe asthma and severe atopic dermatitis, have IgE levels that by far exceeds 200 ng/ml. Resistentia had to close down. However, in academia we decided to continue the project and screened for new, more suitable, targets for a more potent therapeutic allergy vaccine. There was also a greater initiative to focus on adjuvant optimization (93). Three cytokines that act early in the allergic cascade were selected for initial preclinical tests, the potent TH2 inducing cytokines, IL-18, IL-33 and TSLP. These three cytokines have recently been shown to induce severe atopic dermatitis, when over-expressed in the skin, and induction of asthma when administered to the lungs together with an allergen. Such findings indicated that these cytokines are of major importance for the pathogenesis of severe atopic asthma and atopic dermatitis. Species-specific vaccines against these three cytokines were produced for coming tests of efficacy and safety in mice, dogs and humans. Initial tests of the three vaccines in an allergen-induced dermatitis model in mice have recently resulted in very promising data. The IL-33 vaccine resulted in a marked reduction in dermatitis scores (manuscript in preparation). However, the vaccines against TSLP and IL-18 showed very little or no effect. However, these two latter targets may be of importance in combination vaccines. A combination of IL-33 and TSLP may be a potent vaccine for the treatment of asthma. We want to continue the development of the IL-33 vaccine in preclinical models to obtain sufficient information on the safety and efficacy, and to take this vaccine into the clinic. Our plan is to start with dogs with severe atopic dermatitis. However, one major problem is the large costs of clinical trials and the difficulties in getting grants for projects that bridge preclinical and clinical studies.
Adjuvants
In therapeutic vaccine development the adjuvant is a major issue. Tolerance has a strong dampening effect on antibody titers when targeting a self-molecule (Fig 4). This is why potent, safe and biodegradable adjuvants have to be used to gain a clinical effect and to minimize side effects from the vaccine. This was very clear from the clinical trials with the IgE vaccine using alum. Following this knowledge gained from the IgE vaccine project, a lot of time and effort has been devoted by us, in combination with Anna-Karin Olsson’s research group at IMBIM Uppsala University, to develop a safe and potent adjuvant. Such an adjuvant is aimed at being used not only in the development of therapeutic vaccines against allergy, but also for vaccines against autoimmunity and cancer (104,105). A panel of different TLR and NOD agonists were screened in combination with a biodegradable carrier adjuvant, the squalene based Montanide ISA720. Several good candidates were identified (76, 88, 99,107). However, one was superior to all the others; a combination of Montanide ISA 720 and a phosphorothioate stabilized 17 nt long single stranded CpG oligonucleotide. This adjuvant was as good or even better than the ‘golden standard’ in the field; a combination of complete and incomplete Freunds adjuvant (99). We also tested two different oligonucleotides with different sequences and obtained the same results (99). This adjuvant, that is possible to produce under GMP, has been tested with several self-antigens with the continued promising results (99, 107). The adjuvant issue appears to be resolved; we have identified a very good candidate for further clinical trials.
The aim is now to use this adjuvant to continue the preclinical studies in mice and rats. Our intent is to raise funds to enter a toxicity study in contract bred dogs followed by a small preclinical proof of concept study in dogs with atopic dermatitis, one potential future patient population.
People
Lars Hellman, professor
Michael Thorpe, graduate student
Vamsi Boinapally project student
Srinivas Akula project student
Tobias Hein project student
Anna Lena Geiselhöringer project student
Gurdeep Chahal project student
Jing Yu project student
Shen Jiayu project student
Xiaofang Liao project student
Nadine Schmit project student
Joel Svensson project student
Hsun-Yi Hsieh project student
Cherno Sidibeh project student

