Karin Forsberg Nilsson – Exploring brain tumour formation and invasion
Exploring novel regulators of tumour formation and targeting the invasive niche in brain tumours.
Glioblastoma is the most prevalent form of malignant primary brain cancer in adults. Despite therapeutic interventions such as surgical resection, irradiation, and chemotherapy, the median survival for GBM patients remains a mere 15 months. The aggressiveness of GBM is attributed to several factors, including its invasiveness, the presence of abundant and aberrant glioma-associated neovascularization, and the recruitment and accumulation of immune cells that foster an immunosuppressive tumour microenvironment.
Medulloblastoma is a predominantly pediatric brain tumour and it can to some extent be treated to meet the patient’s specific needs, but therapy for the most malignant forms is lacking. In addition, current treatment leads to long-term neurological and sensory consequences, further underscoring the need for improved diagnosis and treatment.
The overall goal of our research is to improve the treatment of malignant brain tumours, in particular glioblastoma (GBM) and medulloblastoma (MB). In our projects we incorporate our experience of neural stem cells with brain tumour biology, and cancer genomics, leveraging these fields to inform novel therapeutic possibilities.
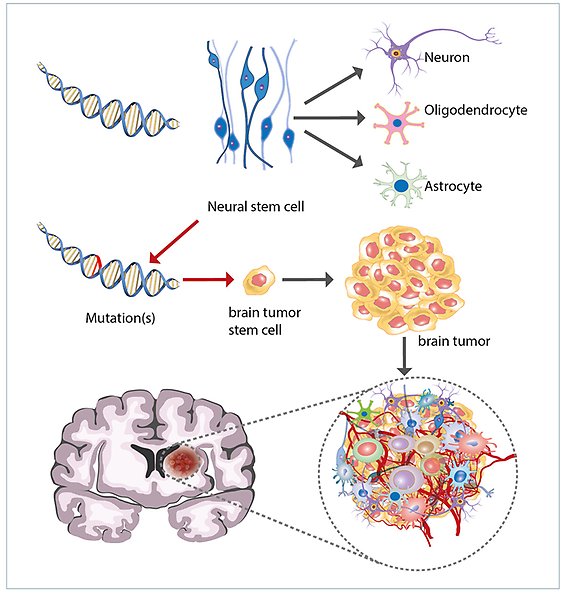
Brain tumours result from mutations in neural stem cells. Neural stem cells normally differentiate to mature cells types of the brain (neurons, astrocytes and oligodendrocytes). If mutations accumulate that lead to a dysregulated growth control, brain tumours may arise.
Leveraging non-coding mutations with evolutionary constraint to discover cancer driver genes in brain tumours
In this project, we explore non-coding mutations in brain tumours to decipher their function. Less than 1.5% of the human genome codes for proteins, and mutations in non-coding regulatory regions (~10%) have largely remained unexplored due to a lack of systematic approaches. We have developed a method to address this, hypothesizing that evolutionary conservation implies function. Leveraging non-coding mutations with scores for evolutionary constraint, we stratify functional mutations from passenger variants.
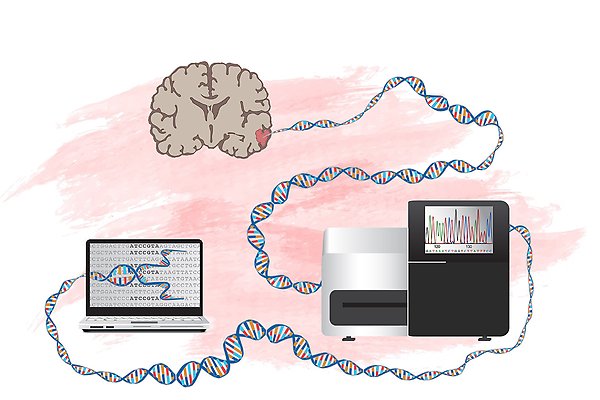
Whole genome sequencing of malignant brain tumours. By sequencing a patient’s entire cancer genome, and comparing to DNA from normal cells of the same individual we can detect all mutations and decipher which ones are important for the disease. Graphic by L Gaffney.
By this new approach we identify and perform functional validation of non-coding mutations with regulatory potential in GBM and MB. We call these mutations non-coding constraint mutations (NCCMs).
In this project we combine whole genome sequencing of brain tumours, the HGCC glioblastoma stem cell repository (established as a collaboration between PIs in the NoDe research programme), and excellent biobanks U-CAN to map NCCMs in cancer of the brain.
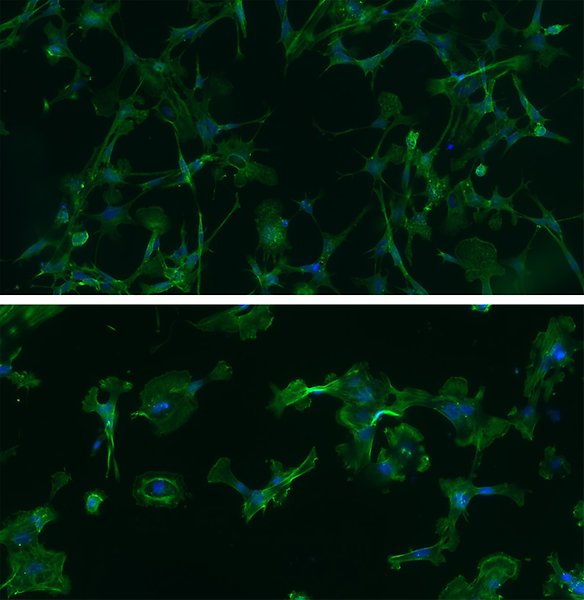
Examples of GBM cells from patients, grown under neural stem cell culture conditions. These cells retain important features of the patient’s tumour and are excellent models to study the disease. Phalloidin staining (green) depicts actin filaments and DAPI (blue) the cell nuclei. Photo: G Wicher.
Targeting the the extracelluar matrix in brain tumours for therapeutic intervention with focus on heparan sulfate proteoglycans
Any tumour stroma outside the brain is usually rich in fibrillar collagens, while in the brain, glycosaminoglycans, glycoproteins and proteoglycans are predominant constituents. Heparan sulfate proteoglycans (HSPGs) are composed of a core protein, to which highly charged, sulfated, disaccharide side chains are attached. The complexity of these side chains is the result of a series of enzymatic modifications that determine their capability to interact with e.g. growth factors. Therefore, remodelling of heparan sulfate (HS) and degradation of HS make up part of the malignant brain tumour signature.
We study HSPG biosynthesis and degradation in clinical brain tumour samples, cell cultures from GBM and medulloblastoma, as well as mouse models of these diseases. We have shown that chemical compounds that inhibit the enzyme heparanase reduces the growth of brain tumour cells. Results from this study are expected to validate and suggest new targets for brain tumour therapy. Possible routes for intervention could be aimed either at inhibiting tumour cell growth or invasiveness.
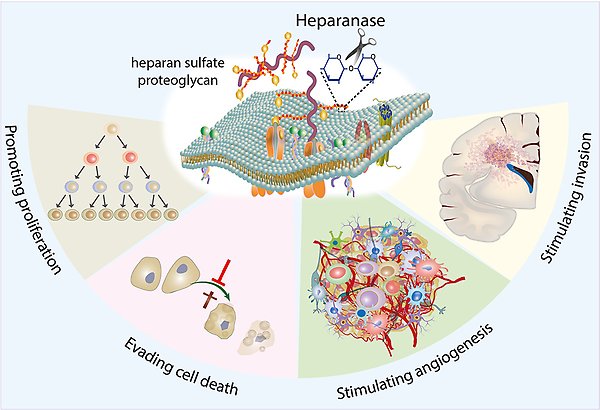
Role of HPSE in brain tumours. Overexpression of heparanase, the enzyme which degrades heparan sulfate proteoglycans, activates signalling pathways that are commonly associated with tumor growth and progression. Herpanase promotes brain tumour cell proliferation, suppresses cell death, stimulates tumour angiogenesis and cancer cell migration and invasion.
The influence of neuroinflammation on brain tumour growth
Knowledge about how the brain responds to a growing tumour remains limited. However, the tumour microenvironment plays a critical role in the progression of the disease. Brain tumours can be viewed as “wounds that can’t stop the healing process”. Drawing parallels between neuro-inflammation and cancer could shed light on the brain's transition to a milieu that fosters tumor growth.
Our research focuses on factors that regulate neuroinflammation in GBM, including the dynamics between tissue-resident cells and immune cells from the peripheral circulation. We examine the role of Interleukin-33 and its receptor, ST2, in shaping the inflammatory microenvironment within glioblastoma.
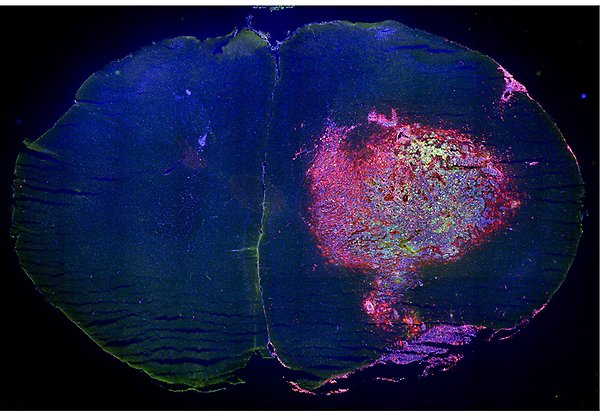
Mouse brain tumour with infiltrated immune cells Coronal section of a mouse brain with a tumour (glioma) in one of the hemispheres. Microglia and macrophages, two types of immune cells, are fluorescently labelled with CD11b in red, while the tumour cells (GL261) are marked with green fluorescent protein. Nuclei are stained with DAPI in blue, providing a comprehensive view of the tumour environment. Photo: G Wicher.
Publications
Part of Acta neuropathologica communications, 2024
- DOI for Decoding of the surfaceome and endocytome in primary glioblastoma cells identifies potential target antigens in the hypoxic tumor niche
- Download full text (pdf) of Decoding of the surfaceome and endocytome in primary glioblastoma cells identifies potential target antigens in the hypoxic tumor niche
Part of Cellular and Molecular Life Sciences (CMLS), 2024
- DOI for Hidden secrets of the cancer genome: unlocking the impact of non-coding mutations in gene regulatory elements
- Download full text (pdf) of Hidden secrets of the cancer genome: unlocking the impact of non-coding mutations in gene regulatory elements
Leveraging base-pair mammalian constraint to understand genetic variation and human disease
Part of Science, p. 367-+, 2023
Live Detection of Neural Progenitors and Glioblastoma Cells by an Oligothiophene Derivative
Part of ACS Applied Bio Materials, p. 3790-3797, 2023
- DOI for Live Detection of Neural Progenitors and Glioblastoma Cells by an Oligothiophene Derivative
- Download full text (pdf) of Live Detection of Neural Progenitors and Glioblastoma Cells by an Oligothiophene Derivative
Using evolutionary constraint to define novel candidate driver genes in medulloblastoma
Part of Proceedings of the National Academy of Sciences of the United States of America, 2023
- DOI for Using evolutionary constraint to define novel candidate driver genes in medulloblastoma
- Download full text (pdf) of Using evolutionary constraint to define novel candidate driver genes in medulloblastoma
Part of Proceedings of the National Academy of Sciences of the United States of America, 2022
PTEN inhibits AMPK to control collective migration
Part of Nature Communications, 2022
- DOI for PTEN inhibits AMPK to control collective migration
- Download full text (pdf) of PTEN inhibits AMPK to control collective migration
Part of Molecular Cancer Research, p. 528-540, 2021
Involvement of Heparan Sulfate and Heparanase in Neural Development and Pathogenesis of Brain Tumors
Part of Heparanase, p. 365-403, Springer, 2020
Part of Cancers, 2020
- DOI for Nuclear Receptor Binding Protein 2 Is Downregulated in Medulloblastoma, and Reduces Tumor Cell Survival upon Overexpression
- Download full text (pdf) of Nuclear Receptor Binding Protein 2 Is Downregulated in Medulloblastoma, and Reduces Tumor Cell Survival upon Overexpression
Part of Genome Biology, 2020
- DOI for Whole-genome sequencing of glioblastoma reveals enrichment of non-coding constraint mutations in known and novel genes
- Download full text (pdf) of Whole-genome sequencing of glioblastoma reveals enrichment of non-coding constraint mutations in known and novel genes
ADAMDEC1 Maintains a Growth Factor Signaling Loop in Cancer Stem Cells
Part of Cancer Discovery, p. 1574-1589, 2019
BET and Aurora Kinase A inhibitors synergize against MYCN-positive human glioblastoma cells
Part of Cell Death and Disease, 2019
- DOI for BET and Aurora Kinase A inhibitors synergize against MYCN-positive human glioblastoma cells
- Download full text (pdf) of BET and Aurora Kinase A inhibitors synergize against MYCN-positive human glioblastoma cells
Heparanase promotes glioma progression via enhancing CD24 expression
Part of International Journal of Cancer, p. 1596-1608, 2019
Part of Oncoimmunology, 2019
- DOI for Human Mesenchymal glioblastomas are characterized by an increased immune cell presence compared to Proneural and Classical tumors
- Download full text (pdf) of Human Mesenchymal glioblastomas are characterized by an increased immune cell presence compared to Proneural and Classical tumors
Part of Cancers, 2019
- DOI for Integrin alpha 10, a Novel Therapeutic Target in Glioblastoma, Regulates Cell Migration, Proliferation, and Survival
- Download full text (pdf) of Integrin alpha 10, a Novel Therapeutic Target in Glioblastoma, Regulates Cell Migration, Proliferation, and Survival
Proteomic approach for understanding milder neurotoxicity of Carfilzomib against Bortezomib
Part of Scientific Reports, 2018
- DOI for Proteomic approach for understanding milder neurotoxicity of Carfilzomib against Bortezomib
- Download full text (pdf) of Proteomic approach for understanding milder neurotoxicity of Carfilzomib against Bortezomib
Reprint of: A chemical screen identifies trifluoperazine as an inhibitor of glioblastoma growth
Part of Biochemical and Biophysical Research Communications - BBRC, p. 136-142, 2018
A chemical screen identifies trifluoperazine as an inhibitor of glioblastoma growth
Part of Biochemical and Biophysical Research Communications - BBRC, p. 477-483, 2017
Part of Matrix Biology, p. 92-104, 2017
Part of Molecular Cancer Therapeutics, p. 1705-1716, 2017
Interleukin-33 Promotes Recruitment of Microglia/Macrophages in Response to Traumatic Brain Injury
Part of Journal of Neurotrauma, p. 3173-3182, 2017
Part of Scientific Reports, 2016
- DOI for ABCG2 regulates self-renewal and stem cell marker expression but not tumorigenicity or radiation resistance of glioma cells
- Download full text (pdf) of ABCG2 regulates self-renewal and stem cell marker expression but not tumorigenicity or radiation resistance of glioma cells
Case-specific potentiation of glioblastoma drugs by pterostilbene
Part of Oncotarget, p. 73200-73215, 2016
Heparanase Promotes Glioma Progression and is Inversely Correlated with Patient Survival.
Part of Molecular Cancer Research, p. 1243-1253, 2016
Open for collaboration: an academic platform for drug discovery and development at SciLifeLab
Part of Drug Discovery Today, p. 1690-1698, 2016
- DOI for Open for collaboration: an academic platform for drug discovery and development at SciLifeLab
- Download full text (pdf) of Open for collaboration: an academic platform for drug discovery and development at SciLifeLab
Part of The European Physical Journal Plus, 2016
Part of PLOS Genetics, 2016
- DOI for Utilizing the Dog Genome in the Search for Novel Candidate Genes Involved in Glioma Development-Genome Wide Association Mapping followed by Targeted Massive Parallel Sequencing Identifies a Strongly Associated Locus
- Download full text (pdf) of Utilizing the Dog Genome in the Search for Novel Candidate Genes Involved in Glioma Development-Genome Wide Association Mapping followed by Targeted Massive Parallel Sequencing Identifies a Strongly Associated Locus
Part of European Journal of Pharmacology, p. 101-107, 2015
Part of Oncotarget, p. 23647-23661, 2015
- DOI for Glioma-derived plasminogen activator inhibitor-1 (PAI-1) regulates the recruitment of LRP1 positive mast cells
- Download full text (pdf) of Glioma-derived plasminogen activator inhibitor-1 (PAI-1) regulates the recruitment of LRP1 positive mast cells
Part of Science Signaling, 2015
Part of EBioMedicine, p. 1351-1363, 2015
- DOI for The Human Glioblastoma Cell Culture Resource: Validated Cell Models Representing All Molecular Subtypes
- Download full text (pdf) of The Human Glioblastoma Cell Culture Resource: Validated Cell Models Representing All Molecular Subtypes
Part of Biochimica et Biophysica Acta - General Subjects, p. 2526-2532, 2014
Part of Acta Physiologica, p. 100-100, 2014
Part of Molecular Oncology, p. 50-58, 2014
Glycosaminoglycans and Glioma Invasion
Part of European Association of NeuroOncology Magazine, p. 75-80, 2014
Heparan sulfate in the regulation of neural differentiation and glioma development
Part of The FEBS Journal, p. 4993-5008, 2014
Rabbit genome analysis reveals a polygenic basis for phenotypic change during domestication
Part of Science, p. 1074-1079, 2014
Selective Calcium Sensitivity in Immature Glioma Cancer Stem Cells
Part of PLOS ONE, 2014
- DOI for Selective Calcium Sensitivity in Immature Glioma Cancer Stem Cells
- Download full text (pdf) of Selective Calcium Sensitivity in Immature Glioma Cancer Stem Cells
Part of Cell, p. 313-328, 2014
Part of PLOS ONE, 2013
- DOI for Adenovirus Serotype 5 Vectors with Tat-PTD Modified Hexon and Serotype 35 Fiber Show Greatly Enhanced Transduction Capacity of Primary Cell Cultures
- Download full text (pdf) of Adenovirus Serotype 5 Vectors with Tat-PTD Modified Hexon and Serotype 35 Fiber Show Greatly Enhanced Transduction Capacity of Primary Cell Cultures
Part of Neuro-Oncology, p. 1469-1478, 2013
Developmental expression of IL-33 in the mouse brain
Part of Neuroscience Letters, p. 171-176, 2013
Interleukin-33 in brain development and traumatic brain injury
Part of Glia, 2013
Intraperitoneal influx of neutrophils in response to IL-33 is mast cell-dependent
Part of Blood, p. 530-536, 2013
Common Denominators of Self-renewal and Malignancy in Neural Stem Cells and Glioma
Part of Stem Cells and Human Disease, p. 387-418, Springer Netherlands, 2012
Part of Restorative Neurology and Neuroscience, p. 9-19, 2012
Neural stem cells: Brain building blocks and beyond
Part of Upsala Journal of Medical Sciences, p. 132-142, 2012
Part of PLOS ONE, 2012
- DOI for Platelet-derived growth factor over-expression in retinal progenitors results in abnormal retinal vessel formation
- Download full text (pdf) of Platelet-derived growth factor over-expression in retinal progenitors results in abnormal retinal vessel formation
Undersulfation of Heparan Sulfate Restricts Differentiation Potential of Mouse Embryonic Stem Cells
Part of Journal of Biological Chemistry, p. 10853-10862, 2012
Part of Journal of Biological Chemistry, p. 24189-24199, 2011
Part of Experimental Cell Research, p. 2779-2789, 2010
2009
Grafted neural progenitors migrate and form neurons after experimental traumatic brain injury
Part of Restorative Neurology and Neuroscience, p. 323-334, 2009
Environmental cues from CNS, PNS, and ENS cells regulate CNS progenitor differentiation
Part of NeuroReport, p. 1283-9, 2008
Part of Molecular and Cellular Neuroscience, p. 32-9, 2008
Stamceller i centrala nervsystemet - Balans mellan tumörutveckling och regeneration
Part of Onkologi i Sverige, p. 29-34, 2008
An activating mutation in the PDGF receptor-beta causes abnormal morphology in the mouse placenta
Part of International Journal of Developmental Biology, p. 361-370, 2007
Enhanced neuronal differentiation in a three-dimensional collagen-hyaluronan matrix
Part of Journal of Neuroscience Research, p. 2138-2146, 2007
Part of Journal of Applied Polymer Science, p. 60-70, 2007
Part of American Journal of Hypertension, p. 743-750, 2007
Part of Developmental Dynamics, p. 2485-2492, 2007
Part of Cancer Research, p. 8042-8048, 2006
Part of NeuroReport, p. 623-628, 2006
Part of Growth Factors, p. 184-196, 2006
Gli1 is not required for Pdgfralpha expression during mouse embryonic development
Part of Differentiation, p. 109-19, 2005
Semicarbazide-sensitive amine oxidase in transgenic mice with diabetes
Part of Biochemical and Biophysical Research Communications - BBRC, p. 1013-1020, 2004
Stem cell factor is a chemoattractant and a survival factor for CNS stem cells
Part of Experimental Cell Research, p. 201-210, 2004
Part of Glia, p. 276-89, 2003
Part of Molecular Cancer Research, p. 147-154, 2002
Immature neurons from CNS stem cells proliferate in response toplatelet-derived growth factor
Part of Journal of Neuroscience, p. 3483-3491, 2001
Platelet-derived growth factor induces chemotaxis of neuroepithelial stem cells
Part of Journal of Neuroscience Research, p. 521-530, 1998
Part of Mechanisms of Development, p. 167-180, 1998
Human mast cells express functional TrkA and are a source of nerve growth factor
Part of European Journal of Immunology, p. 2295-2301, 1997
Development of neuronal precursor cells from embryonic stem cells in vitro
Part of Mechanisms of Development, p. 89-102, 1996
Part of Scandinavian Journal of Immunology, p. 267-272, 1996
Luteal failure in transgenic mice carrying a PDGF dominant-negative mutant/GH hybrid transgene
Part of Transgenics (Basel. Print), p. 515-523, 1995
A divergent effect of PDGF-AA and -BB on differentiating neural stem and progenitor cells
Targeting SOX2 in glioblastoma cells reveals heterogeneity in SOX2 dependency

