Immunotherapy and PET diagnostics in neurodegenerative diseases
In Alzheimer's disease, the protein amyloid-β (Aβ) aggregates and forms insoluble deposits in the brain, often referred to as plaques. However, several studies suggest that Aβ aggregates smaller than plaques, which are still soluble, may be more harmful to the brain than the plaques themselves. For example, it is now believed that the aggregation of Aβ activates the brain's immune cells, leading to widespread and chronic neuroinflammation. Therefore, aggregated forms of Aβ and molecules involved in the neuroinflammatory cascade are important targets for both diagnosis and treatment.
Design of antibodies that cross the blood-brain barrier
Our research is based on antibodies that specifically bind to various forms of Aβ or other proteins related to different disease processes in the brain. An important focus for us is to enable antibodies to cross the blood-brain barrier (BBB), which typically acts as a barrier for large molecules such as antibodies. Therefore, we often modify the antibodies to also bind to the transferrin receptor (TfR), which is expressed on the endothelial cells of the BBB – these antibodies then become bispecific. The endogenous function of TfR is to transport iron to the brain, but we use the receptor as a pathway for antibodies to enter the brain. To optimize the antibodies' pharmacokinetics, distribution, and function, we design bispecific antibodies of various formats and sizes. The antibodies can also be equipped with other types of modifications to influence interactions with other molecules in the brain or to enable the detection of the antibodies.
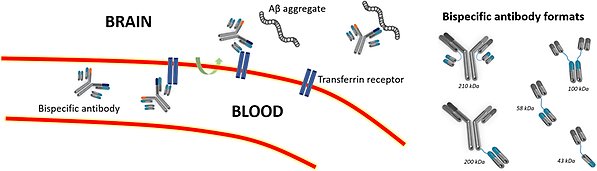
Schematic representation of TfR-mediated transcytosis. The bispecific antibody binds to TfR, facilitating active transport across the blood-brain barrier and into the brain, where the bispecific antibody can bind to its target molecule, such as Aβ aggregates. On the right, bispecific antibodies of various formats and sizes are depicted.
Immunotherapy with next-generation antibodies
In recent years, clinical studies in Alzheimer’s disease patients have shown that antibodies reducing Aβ levels in the brain result in a slower progression of the disease. One such antibody is lecanemab/mAb158, developed in our group. Studies clearly indicate that the clinical effects are better when more antibody reaches the brain. However, administering excessively large doses of antibodies is challenging due to cost considerations and the risk for side effects. Therefore, we are investigating bispecific antibodies that exhibit increased brain uptake through active transport with TfR across the BBB, and we are exploring which properties are necessary for more effective treatment. We particularly focus on understanding the molecular mechanisms behind the antibodies' effects, such as their ability to activate the brain's immune cells to eliminate the aggregated proteins.
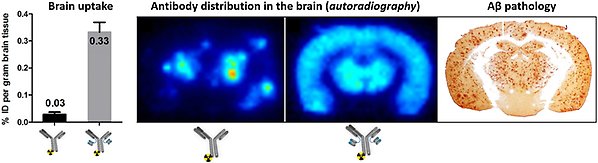
Uptake and distribution of antibodies in the brain. The bispecific antibody exhibits significantly higher uptake in the brain than the "regular" antibody. Moreover, the bispecific antibody evenly distributes across the entire brain, reaching all areas where it can interact with its target molecules, such as Aβ aggregates, as illustrated by autoradiography showing the distribution of radiolabeled antibodies compared to immunostaining of Aβ pathology in the brain.
PET-based diagnostics
As new therapies emerge for Alzheimer's disease, the importance of diagnostics is increasing to identify patients early and to select the right treatment for each individual. The medical imaging technique positron emission tomography (PET) can be used for diagnosis in Alzheimer's disease. Currently, there are several PET radioligands that primarily bind to protein structures that are typical of the "core" of plaques. This type of PET diagnostics generates PET images that clearly indicate whether a person has the characteristic Aβ pathology of Alzheimer's disease in the brain or not.
However, the new immunotherapies are not designed to bind solely to Aβ in the core of plaques but also to other forms of aggregates, which constitute a significant and crucial part of the Aβ pathology causing Alzheimer's disease. Therefore, we aim to develop immunoPET diagnostics based on antibodies that can detect various types of Aβ aggregates, thus better aligning with the new therapies. As the first research group ever, we have demonstrated the use of bispecific antibodies, based on the therapeutic antibodies, as PET radioligands to visualize Aβ aggregates in the brain. We are also investigating whether bispecific antibodies can be used with PET to image other disease-related proteins in the brain, such as proteins linked to neuroinflammation.
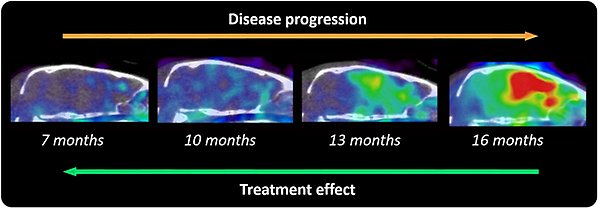
PET study with a bispecific, radiolabeled antibody visualizing the development of Aβ pathology with increased age in Alzheimer's transgenic mice. We have also conducted studies demonstrating the ability of the radiolabelled bispecific antibodies to measure the effects of Aβ-reducing treatments.
We also use established and new PET radioligands to study other changes in the brain during ongoing disease, with a particular interest in investigating how neurons communicate with each other and how this communication deteriorates in neurodegenerative diseases, as well as in normal aging.
Publications
Age, dose, and binding to TfR on blood cells influence brain delivery of a TfR-transported antibody
Part of Fluids and Barriers of the CNS, 2023
- DOI for Age, dose, and binding to TfR on blood cells influence brain delivery of a TfR-transported antibody
- Download full text (pdf) of Age, dose, and binding to TfR on blood cells influence brain delivery of a TfR-transported antibody
Part of Nature Neuroscience, p. 2073-2080, 2023
- DOI for Cryo-EM of Aβ fibrils from mouse models find tg-APPArcSwe fibrils resemble those found in patients with sporadic Alzheimer's disease
- Download full text (pdf) of Cryo-EM of Aβ fibrils from mouse models find tg-APPArcSwe fibrils resemble those found in patients with sporadic Alzheimer's disease
Part of Alzheimer's Research & Therapy, 2023
- DOI for Long-term effects of immunotherapy with a brain penetrating Aβ antibody in a mouse model of Alzheimer's disease
- Download full text (pdf) of Long-term effects of immunotherapy with a brain penetrating Aβ antibody in a mouse model of Alzheimer's disease
Part of Journal of Nuclear Medicine, p. 302-309, 2022
- DOI for 11C-PiB and 124I-antibody PET provide differing estimates of brain amyloid-β after therapeutic intervention
- Download full text (pdf) of 11C-PiB and 124I-antibody PET provide differing estimates of brain amyloid-β after therapeutic intervention
Engineered antibodies: new possibilities for brain PET?
Part of European Journal of Nuclear Medicine and Molecular Imaging, p. 2848-2858, 2019
- DOI for Engineered antibodies: new possibilities for brain PET?
- Download full text (pdf) of Engineered antibodies: new possibilities for brain PET?
Part of Alzheimer's Research & Therapy, 2018
- DOI for Efficient clearence of A beta protofibrils in A beta PP-transgenic mice treated with a brain-penetrating bifunctional antibody
- Download full text (pdf) of Efficient clearence of A beta protofibrils in A beta PP-transgenic mice treated with a brain-penetrating bifunctional antibody
Bivalent Brain Shuttle Increases Antibody Uptake by Monovalent Binding to the Transferrin Receptor
Part of Theranostics, p. 308-318, 2017
- DOI for Bivalent Brain Shuttle Increases Antibody Uptake by Monovalent Binding to the Transferrin Receptor
- Download full text (pdf) of Bivalent Brain Shuttle Increases Antibody Uptake by Monovalent Binding to the Transferrin Receptor
Antibody-based PET imaging of amyloid beta in mouse models of Alzheimer's disease
Part of Nature Communications, 2016
- DOI for Antibody-based PET imaging of amyloid beta in mouse models of Alzheimer's disease
- Download full text (pdf) of Antibody-based PET imaging of amyloid beta in mouse models of Alzheimer's disease
- More publications

