Redox-conductive MOFs
We aim to design redox-conductive MOFs and to understand the influence of all contributing factors.
Our research project
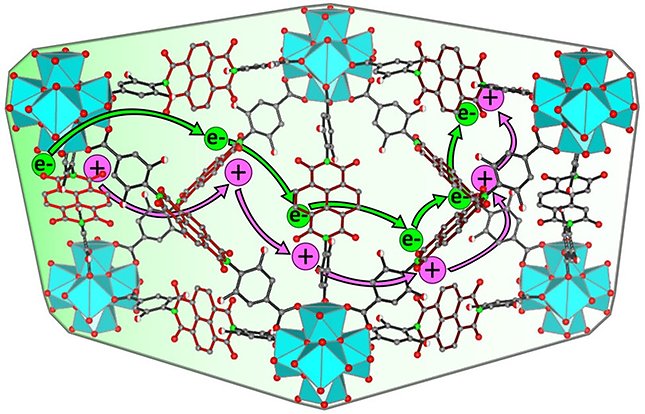
Charge transport through MOFs has two macroscopic mechanisms: band-conduction and redox-conduction. The first pathway relies on sizable orbital overlap (or electronic coupling) between the molecular components of the framework, either with through-bond or through-space approaches. The second pathway takes place via redox-hopping between discrete electroactive sites including redox-active organic linkers (see Figure 1, above), metallo-linkers and open metal sites of the secondary building units.
Figure 1. Redox-conduction between discrete electroactive linkers. Reprinted with permission from J. Am. Chem. Soc. 2022, 144, 5910–5920.
Such redox-conductive MOFs have many potential applications such as energy storage and electrocatalysis, however, both macroscopic and microscopic details of the counterion coupled redox hopping mechanism are still unclear. Our efforts are to design redox-conductive MOFs (Figure 2) and to understand the influence of all contributing factors, such as different counterions, solvent, framework architecture, ligand functionality, external potential, etc.
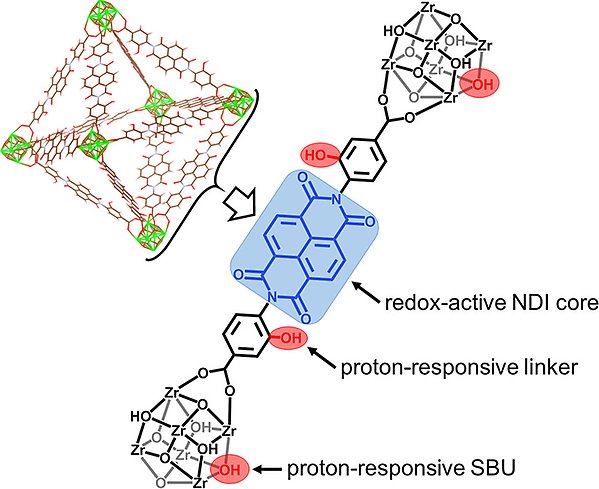
Figure 2. MOF design strategy for developing a redox-active framework featuring UiO topology with proton-responsive functionalities. Reprinted with permission from J. Am. Chem. Soc. 2022, 144, 5910–5920.
Selected References
- Li, J.; Kumar, A.; Johnson, B.A.; Ott, S. Experimental manifestation of redox-conductivity in metal-organic frameworks and its implication for semiconductor/insulator switching. Nat. Commun. 2023, 14, 4388. https://doi.org/10.1038/s41467-023-40110-6
- Kumar, A.; Li, J.; Inge, A. K.; Ott, S. Electrochromism in Isoreticular Metal–Organic Framework Thin Films with Record High Coloration Efficiency. ACS Nano 2023, 17 (21), 21595–21603. https://doi.org/10.1021/acsnano.3c06621
- Castner, A. T.; Su, H.; Svensson Grape, E.; Inge, A. K.; Johnson, B. A.; Ahlquist, M. S. G.; Ott, S. Microscopic Insights into Cation-Coupled Electron Hopping Transport in a Metal-Organic Framework. J. Am. Chem. Soc. 2022, 144, 5910–5920. https://doi.org/10.1021/jacs.1c13377
- Johnson, B. A.; Bhunia, A.; Fei, H.; Cohen, S. M.; Ott, S. Development of a UiO-Type Thin Film Electrocatalysis Platform with Redox-Active Linkers. J. Am. Chem. Soc. 2018, 140, 2985–2994. https://doi.org/10.1021/jacs.7b1307
Cooperation partners
More information to come.
Contact
- If you have any questions about our research, please contact the programme professor Sascha Ott.
- Sascha Ott
