Berger lab: Our research

What we like to talk about in the Berger Lab
Evolution of Condition Dependent Mutation Rates
Mutation provides the fuel for adaptation by generating phenotypic novelty. At the same time most new mutations are deleterious. The mechanisms that shape variation in mutation rate are pivotal in determining the prevalence of mutation load, genetic disease and biological aging, as well as rates of adaptation, speciation and extinction. Until only very recently it was assumed that mutation rate was an invariable, species-specific, parameter, but new evidence has demonstrated considerable intraspecific variation in mutation rates. This novel finding has broad evolutionary implications but empirical explorations of the underlying causality and impacts at the population level have not been well studied in diploid organisms.
In the Berger lab we aim to explore the basis for, and demographic consequences of, condition dependent mutation rates in sexually reproducing organisms. Specifically, we apply a life history theory framework to understand variation in mutation rate as a function of the total amount of resources carried by individuals, and a trade-off between allocating those resources to DNA repair or competing biological functions.
In this research we use the seed beetle Callosobruchus maculatus but also other laboratory organisms such as fruit flies and nematode worms, all three amendable model systems for life history and sexual selection research. We combine experimental evolution, mutation induction and mutation accumulation approaches with genome sequencing, allowing experimental comparative genomics to understand variation in mutation rate and its evolutionary consequences.
The research has several related and overarching foci:
- Does environmental stress lead to increased germ line mutation rates via compromised DNA repair by lowering the phenotypic condition of individuals?
- How does environmental stress modulate the putative trade-off between investment in reproduction, survival and germ line DNA repair?
- How does DNA repair efficiency of the germ line (and consequential mutation rates) evolve during the process of compensatory adaptation to stressful conditions?
- What are the effects of condition dependent mutation rate on evolution under different life histories and mating systems?

Our main weapon of choice, the seed beetle Callosobruchus maculatus. Left: A female laying eggs on a black eyed bean. Right: a mating couple, male to the back/right, female to the front/left. Photo credit: Ivain Martinossi-Allibert.
Mutational Effects of Selection Past
The study of mutational effects has a long history in biology and is key to understanding evolutionary processes. Recent modelling and novel laboratory experiments suggest that the force of selection may itself mould underlying genetic architectures to become more robust to both environmental and genetic perturbations, thereby potentially moderating phenotypic effects of novel mutations. Such a feed-back loop between selection and mutation challenges the classical view of mutational effects as deterministic and independent of the forces of natural selection, and thus necessitates a more developed theory of evolution. However, despite the progress in our understanding of these dynamics, known examples of how selection for genetic robustness of developmental systems has affected mutational effects in natural populations are lacking.
In the Berger lab we aim to demonstrate that past natural selection has played a decisive role in shaping genetic architectures to modify the effects of both novel mutations and standing genetic variation, based on experimental comparisons in two separate natural systems. We collaborate with Prof. Karl Gotthard (Stockholm University) working on the speckled wood butterfly, Pararge aegeria. This butterfly expresses alternative developmental pathways in different geographical regions, but all pathways can be experimentally induced in the laboratory in all populations, allowing the study of mutational effects in pathways that have either been under selection, or relaxed selection, for long evolutionary time frames in nature. Secondly, we are collaborating with Prof. Frank Johansson (Uppsala University) and Prof. Phill Watts (Oulo University) to study quantitative genetic covariation linking gene expression to multivariate phenotypes in damselfly populations transplanted across latitude along two replicated clines, allowing us to study the link between molecular and phenotypic robustness (and its converse; the release of cryptic genetic variation) across ancestral and novel environments.
Our aim is to provide an important step forward in our general understanding of the evolution of genetic architecture and how natural selection, as a direct or indirect consequence, modifies fitness effects and pleiotropy of de novo mutations.

Same-sex mounting behavior in male seed beetles. Our research has shown that intralocus sexual conflict can increase same-sex sexual behaviors in males due to the genes underlying them having benefits when expressed in females (Berger et al. 2016; BMC Evol Biol). Photo credit: Ivain Martinossi-Allibert.
Sex and Environment Specific Selection and Adaptation
The consequences of sex- and environment-dependent mutation rates are contingent on their fitness effects. If stress increases selection on new mutations, adaptation should be more efficient in new environments, but neither theory nor empirical data supports a general increase in selection under stress. Moreover, despite being of fundamental importance for rates of adaptation in sexual populations, we know very little about sex-specific changes in the strength of selection under stress.
The evolution of sex and the consequences of sex-specific selection is one of the most researched areas in evolutionary biology and currently very fascinating and vibrant. Intralocus sexual conflict (ISC) has been a central theme in this research over the last decade. ISC occurs because even though males and females share the same genome, they often experience distinct selection pressures. This leads to a genetic conflict where genes conferring a fitness advantage to one sex inflict costs when expressed in the other. Such sexually antagonistic genes can impose a substantial fitness load in sexual populations, and affects a range of fundamental processes such as the evolution of sexual dimorphism and genetic architecture, the maintenance of genetic variation, and rates of adaptation. Theory suggests that in well-adapted populations most of the mutations that can contribute to adaptive evolution will have sexually antagonistic fitness effects, putting further adaptation to a halt. However, new mutations are predicted to have less sexually antagonistic effects in maladapted populations, where adaptation should matter the most.
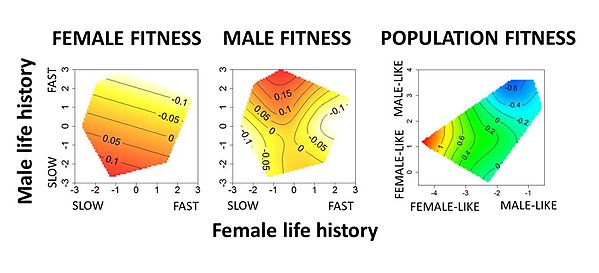
Intralocus sexual conflict over optimal life history and its population consequences in the seed beetle Callosobruchus maculatus. Female genotypes with a slow life history (long development, low metabolic rate and large body size) have high reproductive success ( left), but the same genotypes expressed in males show low reproductive success (middle) as they instead benefit from a fast life history. Population constructs of the genotypes show that populations composed of genotypes with high male (but low female) fitness have lower productivity and survival. Figure modified from Berger et al. 2016; American Naturalist).
Understanding how sex-specific selection acts across the genome over spatio-temporal scales is thus essential for predicting evolution and adaptation in sexually reproducing populations, and one of the central themes of our ongoing research. We collaborate with the group of Prof. Göran Arnqvist at Uppsala University to employ a range of empirical strategies using seed beetles, including sex-limited artificial selection and mutation accumulation, combined with conceptual models incorporating fitness landscape theory, to explore the role of sexual selection and genetic conflict during adaptation to new environments.
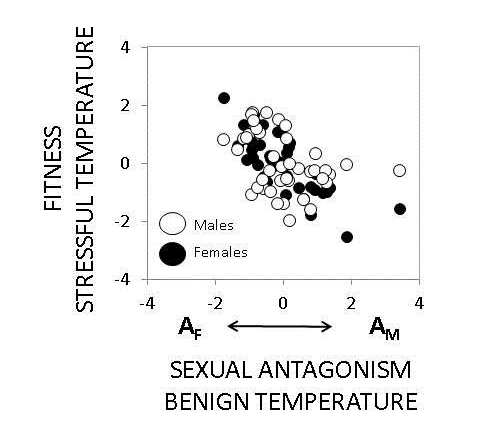
The role of the environment in modulating intralocus sexual conflict in a population of C. maculatus. Under benign temperature regimes, male and female reproductive success are negatively genetically correlated. However, under temperature stress, this correlation becomes positive. The underlying reason for this shift seems to be that sexually antagonistic gene variants with negative effects on females but positive effects on males under normal temperatures (values towards AM on x-axis) seem to have very deleterious effects in both sexes at stressful temperature (values on y-axis). Figure modified from Berger et al. 2014; Evolution.
