In vitro bioassays för pro- och retrospektiv miljötoxicitetsprövning.
Description
De novo establishment of in vitro fish bioassays via CRISPR/Cas-mediated reporter gene knock-ins for pro- and retrospective environmental toxicity testing. Project leader: Sebastian Lungu Mitea
Pro- and retrospective regulatory toxicity testing accounts for approximately 25% of animals used in research. Besides rodents, fish are the second most applied model. Efforts have been undertaken within human toxicology to transform the discipline – from a lethality-driven to a mechanistically-driven one. Many new approach methods, including in vitro and in silico test systems, were developed. However, within ecotoxicology, this process is in its infancy. Many mammalian and bacterial bioassay were adapted to compensate for the latter but lack ecological relevance and are technically problematic. Recent studies disclosed that cellular reporters derived via classic gene-engineering and transgenesis technologies might be subject to artefacts.
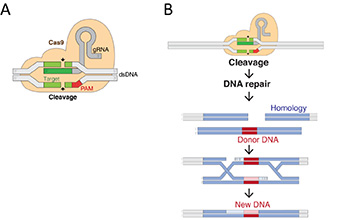
Contrarily, the CRISPR/Cas tools avoid such pitfalls, given their high resolution and accuracy. Here, we use this technology to design novel reporter gene assays to assess primary toxicity pathways in zebrafish cell lines. The idea is to drive reporter gene expression by endogenous genomic regulatory elements within their original loci. Thereby, an actual physiological response can be recorded without drastically alternating the cellular system under investigation.
Further, the approach is supplemented by computational modeling, such as mass balance equations and in vitro to in vivo extrapolations. Modelling is utilized to compute actual bioavailable effect concentrations in vivo from nominal exposure concentrations in vitro. Thereby, regulatory acceptance could be fulfilled, leading to the partial or total replacement of acute and chronic regulatory in vivo testing in an ecotoxicological context. We regard such in vitro assays as a technological improvement within the field of ecotoxicology, with the potential to drastically decrease the use of fish in regulatory toxicity testing.
This project has received 2 million SEK from the U-share project at the disciplinary domain of medicine and pharmacy, UU and will be conducted in a collaboration with colleagues from SLU (molecular toxicology) and KI (genome engineering lab).
Selected publications
- Lungu-Mitea S, Lundqvist J. Potentials and pitfalls of transient in vitro reporter bioassays: interference by vector geometry and cytotoxicity in recombinant zebrafish cell lines. Arch Toxicol. 2020 Aug;94(8):2769-2784. doi: 10.1007/s00204-020-02783-6. Epub 2020 May 23. PMID: 32447522; PMCID: PMC7395025
- Lungu-Mitea S, Han Y, Lundqvist J. Development, scrutiny, and modulation of transient reporter gene assays of the xenobiotic metabolism pathway in zebrafish hepatocytes. Cell Biol Toxicol. 2021 Oct 15. doi: 10.1007/s10565-021-09659-0. Epub ahead of print. PMID: 34654992.
- Lungu-Mitea S, Vogs C, Carlsson G, Montag M, Frieberg K, Oskarsson A, Lundqvist J. Modeling Bioavailable Concentrations in Zebrafish Cell Lines and Embryos Increases the Correlation of Toxicity Potencies across Test Systems. Environ Sci Technol. 2021 Jan 5;55(1):447-457. doi: 10.1021/acs.est.0c04872. Epub 2020 Dec 15. PMID: 33320646; PMCID: PMC7872314.
Publications
Part of Nucleic Acids Research, 2024
- DOI for Visualizing DNA single- and double-strand breaks in the Flash comet assay by DNA polymerase-assisted end-labelling
- Download full text (pdf) of Visualizing DNA single- and double-strand breaks in the Flash comet assay by DNA polymerase-assisted end-labelling
Part of PLOS ONE, 2024
- DOI for Screening for antibacterial and cytotoxic activities of Sri Lankan marine sponges through microfractionation: Isolation of bromopyrrole alkaloids from Stylissa massa
- Download full text (pdf) of Screening for antibacterial and cytotoxic activities of Sri Lankan marine sponges through microfractionation: Isolation of bromopyrrole alkaloids from Stylissa massa
Genotoxicity study of Ethiopian medicinal plant extracts on HepG2 cells
Part of BMC Complementary and Alternative Medicine, 2018
- DOI for Genotoxicity study of Ethiopian medicinal plant extracts on HepG2 cells
- Download full text (pdf) of Genotoxicity study of Ethiopian medicinal plant extracts on HepG2 cells
DNA integrity under alkaline conditions: An investigation of factors affecting the comet assay
Part of Mutation research. Genetic toxicology and environmental mutagenesis, 2023
- DOI for DNA integrity under alkaline conditions: An investigation of factors affecting the comet assay
- Download full text (pdf) of DNA integrity under alkaline conditions: An investigation of factors affecting the comet assay
Part of Exposure and Health, p. 547-554, 2020
- DOI for Evaluation of Potential DNA-Damaging Effects of Nitenpyram and Imidacloprid in Human U937-Cells Using a New Statistical Approach to Analyse Comet Data
- Download full text (pdf) of Evaluation of Potential DNA-Damaging Effects of Nitenpyram and Imidacloprid in Human U937-Cells Using a New Statistical Approach to Analyse Comet Data
Part of Mutation research. Genetic toxicology and environmental mutagenesis, 2020
Myrcene Attenuates Renal Inflammation and Oxidative Stress in the Adrenalectomized Rat Model
Part of Molecules, 2020
- DOI for Myrcene Attenuates Renal Inflammation and Oxidative Stress in the Adrenalectomized Rat Model
- Download full text (pdf) of Myrcene Attenuates Renal Inflammation and Oxidative Stress in the Adrenalectomized Rat Model
Part of Phytotherapy Research, p. 507-514, 2013
Part of Toxicology, p. 57-64, 2009
An analysis of Vigimed, a global E-mail system for the exchange of pharmacovigilance information
Part of Drug Safety, p. 883-889, 2007
Part of MethodsX, 2020
In vitro bioanalysis of drinking water from source to tap
Part of Water Research, p. 272-280, 2018
Part of Planta Medica, 2016
Part of Toxicology in Vitro, p. 716-722, 2007
Part of Toxicology in Vitro, p. 266-271, 2009
Potential genotoxicity of plant extracts used in Ethiopian traditional medicine
Part of Journal of Ethnopharmacology, p. 136-142, 2009
Part of Cell Biology and Toxicology, p. 401-411, 2007
Pharmacokinetics in Mouse and Comparative Effects of Frondosides in Pancreatic Cancer
Part of Marine Drugs, 2016
- DOI for Pharmacokinetics in Mouse and Comparative Effects of Frondosides in Pancreatic Cancer
- Download full text (pdf) of Pharmacokinetics in Mouse and Comparative Effects of Frondosides in Pancreatic Cancer
Fungicide prochloraz induces oxidative stress and DNA damage in vitro
Part of Food and Chemical Toxicology, p. 36-41, 2016
Genotoxicity and Cellular Uptake of Cyclotides: Evidence for Multiple Mode of Action
Part of Mutation research. Genetic toxicology and environmental mutagenesis, p. 176-181, 2012
Part of Free radical research, p. 692-698, 2013
Part of Mutagenesis, p. 637-644, 2013
Frondoside A enhances the antiproliferative effects of gemcitabine in pancreatic cancer
Part of European Journal of Cancer, p. 1391-1398, 2014
Part of International Archives of Occupational and Environmental Health, p. 185, 1997
Part of J Occupational Health, p. 198, 1998
Part of Mutation Research, p. 43, 2003
Part of Exp Oncol, p. 102-7, 2005
Part of Toxicology and applied pharmacology
Part of Mutation Research, p. 43-55, 2003
Part of Toxicology In Vitro, p. 779-786, 2005
Part of Toxicological Sciences, p. 162-170, 2003

