Advanced materials characterisation

We use state-of-the-art methods within local, national and international research infrastructure to characterize synthesized materials and their properties.
Our research
High-quality materials research cannot be performed without thorough materials charactersisation to determine crystal structure, chemical composition and microstructure. It is thus natural that a research programme with focus on the development of new materials and synthesis processes develops considerable competence and experience in materials analysis. Researchers from inorganic chemistry routinly use state-of-the-art methods to characterise the synthesised materials, and their properties. For this we use local, national and international research infrastructure. Below we highloght our activities connected to spectroscopy, neutron and X-ray scattering, as well as electrochemical methods..
Advanced spectroscopic techniques
The toolbox of advanced spectroscopic techniques that we use within the Inorganic chemistry research programme includes XPS, HAXPES, NEXAFS, XANES and EXAFS, most of which are performed using synchrotron radiation at various facilities. XPS can detect all elements except hydrogen, as well as their oxidation state. This gives information of the chemical environment of each element in a sample. The method is surface sensitive, but the information depth can be varied by changing the energy of the incoming X-rays. More energetic X-rays (hard or tender X-rays as in HAXPES) gives more bulk sensitive measurements and can therefore be used to study the material below a thin surface oxide in a non-destructive way. Less energetic X-rays (soft X-rays) is on the other hand sensitive to the outermost surface and is therefore used to study changes to the surface induced by e.g. electrochemical treatments. The X-ray absorption techniques NEXAFS, XANES and EXAFS are also sensitive to oxidation states and can give element-specific information about the short-range order in a material.
Examples of scientific questions that we address with a combination of spectroscopic techniques includes:
- How much charge transfer is there between the metals in a high-entropy alloy?
- Is there any preferential sites for interstitial elements in multicomponent materials
- What happens, at an atomic level, when we corrode a multicomponent material?
For more information, contact Ass. Prof. Erik Lewin
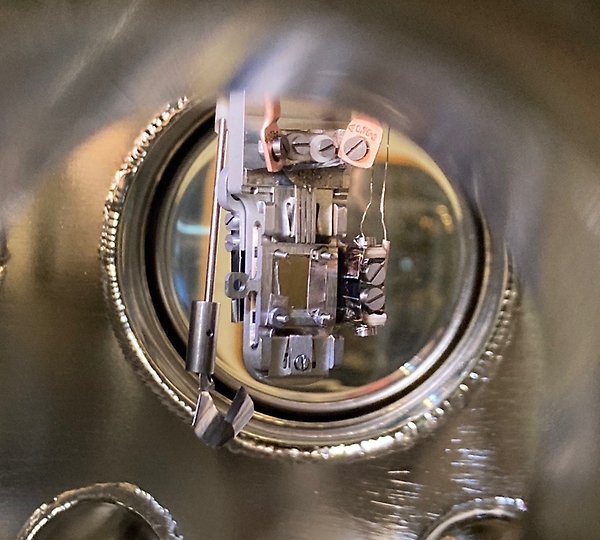
Neutron scattering experiments
Within the Inorganic chemistry research program, neutron scattering is used to obtain crystallographic information that is not possible to attain through X-ray methods. The neutron is unique in how it interacts with matter, which makes it possible to distinguish properties invisible to other probing tools, such as elements/ions with similar or equal number of electrons, and finding elements with low atomic number in a matrix of heavy elements. The ability to extract information about magnetic structure and properties is another stronghold. The strong suite of neutron scattering instruments at the ISIS neutron spallation source, not the least powder diffractometers makes ISIS the primarily used facility. Also, the high penetration power of neutrons is an advantage when performing studies involving complex sample chambers for in-situ/ operando studies.
You can read more about these methods at the Centre for Neutron Scattering.
Contact: Prof. Martin Sahlberg, och Prof. Paul Henry
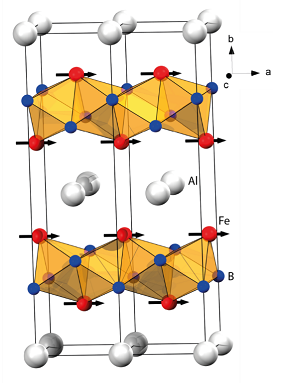
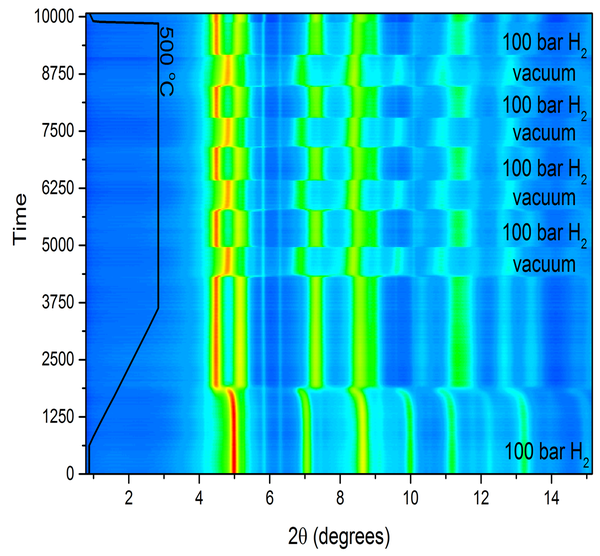
X-ray scattering at synchrotron facilities
The access to high brilliance X-ray sources is critical for the crystallographic studies performed within the research programme. Beamline P02.1 at PETRA III, DESY in Hamburg is routinely used for in situ time resolved studies with controlled temperature, high hydrogen pressure (up to 300 bar) as well as under magnetic fields (up to 1 T). The figure shows an example of phase transformations and cycling stability of a HEA-hydride at 500 °C, showing the in-situ diffractograms (to the left) and the weight percentage of the 2 phases plotted against time (to the right).
Contact: Prof. Martin Sahlberg
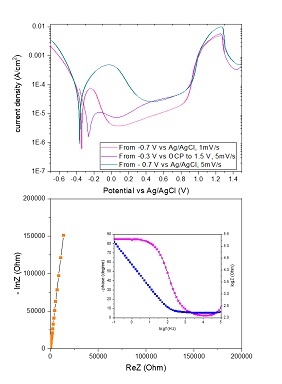
Advanced electrochemical methods
Electrochemistry deals with chemical reactions which involve either the consumption or the production of electricity. Typical electrochemical examples include batteries, fuel cells and electrolysis which involve redox (reduction and oxidation) reactions. In the electrochemistry laboratory of the Inorganic Chemistry research program, several electrochemical methods such as potentiostatic techniques (i.e., chronoamperometry), potentiodynamic techniques (i.e., cyclic voltammetry, polarization curves), and electrochemical impedance spectroscopy are used to obtain information regarding the electrochemical behavior of the studied materials.
Contact: Prof. Leif Nyholm
Contact
- If you have questions about our research, you are welcome to contact the programme professor Professor Martin Sahlberg.
- Martin Sahlberg
