Artificial photosynthesis and solar fuel
If we could capture solar energy and store it in the form of a fuel – a solar fuel – we could satisfy a large part of our energy needs.
Our research
During one hour, the earth receives as much solar energy as humans consume in a year. But we need to be able to store solar energy in concentrated form to be able to use it in e.g. vehicles, and during the night and winter when we have less sunlight. If we could capture solar energy and store it in the form of a fuel – a solar fuel – we could satisfy a large part of our energy needs. A solar fuel can be e.g. hydrogen or an alcohol made from just water and carbon dioxide, using solar energy.
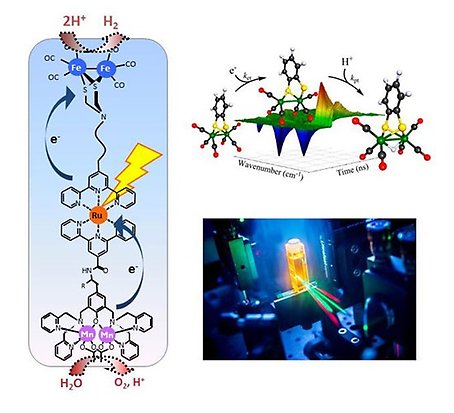
One way to make solar fuel is artificial photosynthesis. They are artificial systems with molecules and materials that mimic the principles of green plant photosynthesis. In the end, these can be more energy efficient than nature. They capture sunlight and use the light's energy to split water into oxygen and electrons. The electrons are then used to reduce carbon dioxide and protons to a fuel, such as hydrogen or an alcohol.
In photosynthesis, light is captured by pigments such as chlorophyll, and the light energy is used to move electrons from molecule to molecule. The electrons are then used to reduce carbon dioxide to carbohydrates, which are an energy-rich "fuel". New electrons are fed into the chlorophyll by splitting water into oxygen, which we breathe. This is a powerful recipe for converting water and carbon dioxide into oxygen and fuel using solar energy. But the plants use much of the energy for their life processes, so the production of biomass is not that efficient. If we can instead do the corresponding process in an artificial system, we could achieve a very high energy efficiency in the conversion to a fuel.
In the project, we study artificial molecular systems that capture light, transfer electrons and use these to split water and make a fuel. We study how to control the transfer of electrons and protons between molecules. Splitting water and making a fuel requires moving several electrons and protons at the same time. It can be compared to juggling many balls at the same time. An important part of the project is to develop molecular catalysts, which can "juggle" in a fast and energy-efficient way.
The project is carried out in a large collaboration within the Swedish Consortium for Artificial Photosynthesis, as well as in collaboration with international research groups.
Contact
- If you have questions about our research, you are welcome to contact programme professor Leif Hammarström.
- Leif Hammarström
