Iron-manganese cofactors in dimetal carboxylate proteins
Why two different metals instead of one?
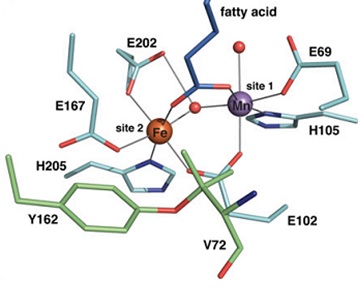
Metal containing cofactors are present in the active sites in many proteins. The presence of metal ions gives access to a wide variety of chemical reactions that would otherwise be difficult to carry out. One superfamily of iron containing proteins has two iron ions in the active site, predominately coordinated by carboxylate groups (from glutamate and aspartate side chains) and imidazole groups (from histidine side chains). This active site motif has been called the diiron-carboxylate cofactor, or more recently the dimetal-carboxylate cofactor. The most studied members of this family of dimetal-carboxylate proteins are the bacterial multicomponent monooxygenases (BMMOs), especially soluble methane monooxygenase (sMMO), and ribonucleotide reductase R2 proteins (RR2s).
Recently Fe-Mn varieties of dimetal-carboxylate protein were found. One of these Fe-Mn enzymes, an oxidase from Mycobacterium tuberculosis, is an R2 homologue but closely related to BMMOs and is capable of C-H activation. Another Fe-Mn enzyme is an RR2 from Chlamydia trachomatis that store a radical equivalent as a MnIV-FeIII species. Even a Mn-Mn variety of an RR2 protein has been reported that retains the function of R2 proteins but require additional flavodoxin to generate an oxidant. The suggested mechanisms for the Fe-Mn and Mn-Mn proteins involve formation of high-valent metal ions (FeIII, FeIV, MnIII and MnIV) in important intermediates, similar to the mechanism suggested for sMMO.
Together with groups at Stockholm University, Freie Universität Berlin, and Technische Universität Kaiserslautern we are involved in a project studying the Fe-Mn varieties of dimetal-carboxylate protein. We are especially interested in questions regarding metal specificity. How does the enzyme know which metal to bind, and how can it distinguish between the two? Also, why does the enzyme bind two different metals? What type of chemistry is the heterometallic cofactor able to perform that the homometallic cannot do?
Figure above: The active site of a Fe-Mn containing R2 homologue from Mycobacterium tuberculosis..
Project: Iron-Manganese Complexes as Biomimetic Models for a New Class of Dimetal Carboxylate Proteins
This project is in collaboration with Sascha Ott.
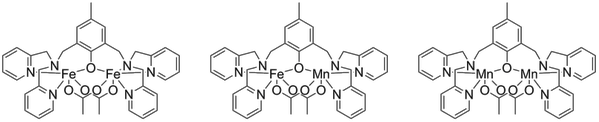
In this project we are synthesizing and characterizing heterometallic complexes that contain iron and manganese. We are particularly interested in issues regarding metal specificity and differences in reactivity between heterometallic and homometallic dinuclear complexes. The aim is to study the reactivity of similar complexes with Fe-Fe, Fe-Mn, and Mn-Mn cores in aqueous environment using a combination of electrochemistry and spectroscopy.
Figure below: A set of complexes with Fe-Fe, Fe-Mn and Mn-Mn cores studied in the group.
Cooperation partners
More information to come.
