Water oxidation
Water oxidation – the oxidative side of Artificial Photosynthesis
The aim of artificial photosynthesis is to capture solar energy and store it in chemical bonds. For this purpose the natural photosynthesis serves as model and an inspiration. Natural photosynthesis uses the energy in sunlight to oxidatively split water to oxygen, protons and electrons. The reducing power of these electrons can be stored in various products to be used by the organism as a fuel when needed.
Artificial photosynthesis aims at mimicking these reactions and store the energy from sunlight in a chemical fuel (a solar fuel). A longstanding goal of artificial photosynthesis has been to develop water oxidation catalysts, based on first row transition metals, which operates at low over potential and can be driven by sunlight. These catalysts will be essential in the process to make a renewable solar fuel.
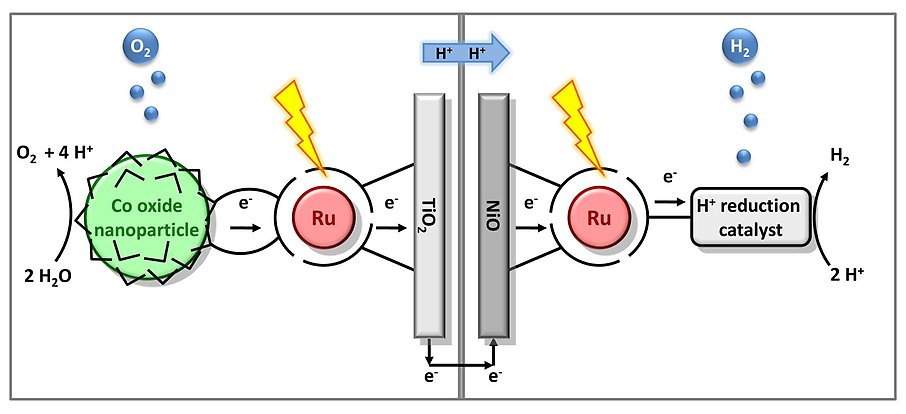
In order to utilize the water oxidation catalyst in a set-up that uses sunlight and water to produce a fuel the water oxidation catalyst has to be coupled to a reduction catalyst that makes the fuel. If protons (released from the water oxidation) are reduced to form hydrogen we get a high energy fuel that does not require any additional starting materials. If we use other reduction catalysts we can also envisage producing other fuels, for example methanol from CO2 and protons, using the same oxidative half-cell. It can be beneficial to have the complete system divided in two compartments. A two compartment solution allows for easy separation of the resulting products and makes internal tuning of the catalysts possible.
Figure above: A schematic representation of a complete cell for light driven water oxidation and hydrogen production. To the left is the oxidative half cell that is the main focus of this project. To the right is the reductive, hydrogen producing, half cell.
Within artificial photosynthesis, we are currently working on the following projects:
Cooperation partners
More information to come.
