Research projects in Emanuelsson Group
Our research focuses on two main themes, electrification of organic polymers and selective adsorption of metal ions from complex aqueous solutions. The research is supported by the Research Council Formas, the Åforsk Foundation, the Magnus Bergvalls Foundation, the Lars Hiertas Memorial Foundation and Uppsala University.
Electrifying organic frameworks
The prevalence and structural diversity of organics together with the possibility for tuning properties through organic synthesis makes organic materials promising candidates for a range of future functional materials. Electrocatalysis and energy storage are two crucial technologies needed to advance a greener and more circular society in which organics could play an important role. For these applications a redox active unit that allows reversible oxidation and reduction is needed. Immobilizing such a structure as part of a polymeric material endows it with both robustness and stability normally only found in inorganics. Crucially, the redox unit inside the polymeric structure benefits from high porosity, resulting in exposed redox sites which allows efficient interaction with e.g. ions or reactants.
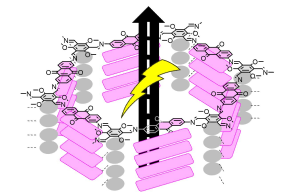
Organic frameworks (e.g. metal organic frameworks - MOFs or covalent organic frameworks - COFs) are polymeric and microcrystalline network compounds formed through self-assembly of two small building blocks. Under the right conditions polymeric structures extending in two or three dimensions with extensively porous structure are formed. These materials possess large surface areas with apparent BET area in the order of thousands of square metres per gram. Incorporating redox units as part of the framework, forming redox active organic frameworks, is therefore a promising strategy for energy storage or electrocatalysis. However, to function in these applications two fundamental challenges needs to be overcome. Firstly, the material needs to be electrically conducting as the redox centres require efficient transport of electrons to function. Secondly, at the same time, there needs be an efficient way for molecules and ions to diffuse through the material in order to sustain the redox reactions. A general and efficient strategy to accomplish this is lacking today. Mixing with ill-defined macroscopic conductive carbons is prevalent method, but for porous materials this approach is often problematic and result in low redox site utilization.
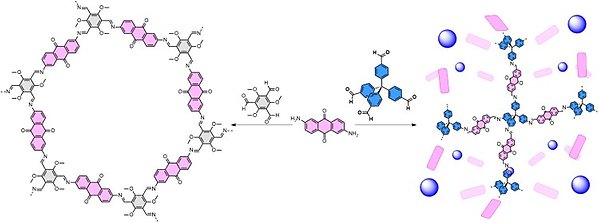
In our research we explore general routes for electrifying organic frameworks that allow any and all redox-active organic frameworks to be employed and tested as electroactive functional materials. This can be achieved by making the framework conductive, or alternatively, electrification can be achieved by forming conducting pathways within the porous void to yield composites. We also attempt to rationalize what types of polymeric material are best suited for different applications as a way to guide further studies into this area.
The research combines synthetic organic chemistry to produce novel redox-active building blocks and polymerization into organic frameworks with materials characterization (e.g. powder XRD, BET surface area, TGA) and electrochemistry (three and two electrode setups) to explore fundamental properties as well as applications. See the below articles for some examples of our previous work on electrification using chains of 1D heteroaromatic polymers and applications towards organic batteries:
J. Mat. Chem. A, 2023, 11, 13923
ACS Appl. Mater. Interfaces, 2021, 13, 5349
Selective capture of metal-ions
Achieving a green and circular society requires a shift from a linear economy, where resources are extracted, used, and then discarded, to a circular model that maximizes the use of resources and minimizes waste. Current mining practices only extract the most economically favorable materials and leave the processed residues as waste. This despite that the residues often contains substantial amounts of a range of metals that have been excavated and processed at high environmental and economic cost. Many billions of tons of such waste are deposited as ashes, muds or sludges all around the world. Emerging technologies challenge this and offer better utilization of excavated materials, but extraction and separation are challenging due to the complex mixture of elements in the waste. In this project we explore how porous organic materials can be tailored to selectively adsorb and subsequently release metal-ions from aqueous solutions. We explore both passive as well as active adsorption targeting metal-ions of high societal importance and try to establish what types of functionality and polymeric structures can achieve this. Thus, we collaborate with industrial stakeholders on industrial needs and what possibilities exists.
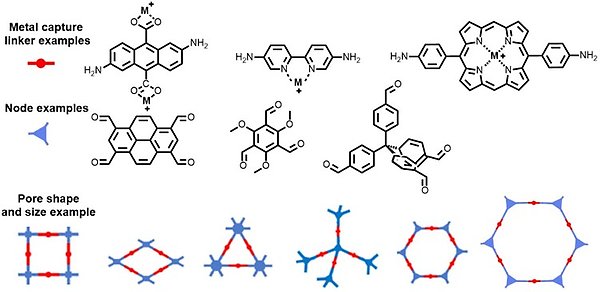
The project combines synthetic organic chemistry to produce building blocks and polymerization into porous polymers. We also employ post-synthetic modifications to introduce or alter the polymer and characterize its properties. Metal-ion adsorption capability is evaluated using both lab mixtures of relevant metal-ions as well as real-world solutions from our industrial partners.
