Research projects in Kihlberg Group
Research in chemical biology and biomedicine is a cornerstone for increasing our understanding of how diseases arise, and thus also for our ability to develop new drugs. Models in which diseases can be studied in molecular and cellular systems as well as in laboratory animals are central to biomedical research. The lack of models that mimic the course of disease in humans is an important explanation for the problems that have affected the pharmaceutical industry in recent years.
In chemical biology, so-called chemical tools are developed, i.e. compounds designed to affect a target molecule. The target molecule is often a protein that is suspected of being involved in the course of a disease. Through the use of chemical tools in model systems, hypotheses about disease progression can be evaluated and ideas for new drugs can be born. We believe that too narrow a focus on developing only chemical tools with properties similar to those that already exist is a further explanation for the problems in developing new drugs. Research with chemical tools also provides generally useful knowledge in the field of molecular signalling, i.e. how molecules affect each other. Fabrication of chemical tools requires good knowledge of organic chemistry as the methodology in this area is constantly evolving. In addition, experience of how the chemical tools should be designed to bind specifically to the target molecules is required. It also requires an understanding of how the chemical tools are absorbed in an organism, as well as how they are distributed to different organs, metabolized and excreted.
This research program aims to develop new chemical tools, especially those that lie outside the areas in which the pharmaceutical industry traditionally works. The tools will then be used in biomedical collaborative projects with access to animal models with a recognized good correlation to humans. The programme has three sub-projects, one of a more fundamental nature and two focused on understanding complex diseases.
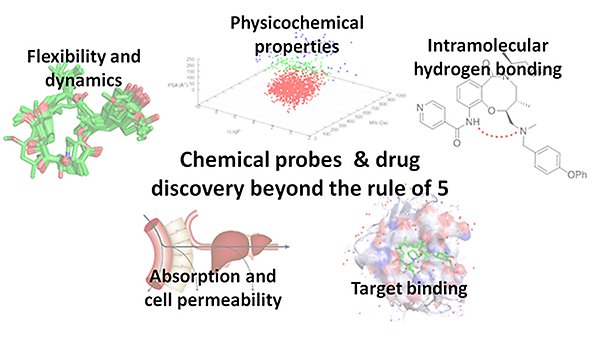
Expanding chemical space beyond the "rule of 5"
Drugs in chemical space beyond Lipinski's rule of 5 (bRo5) have been avoided by the pharmaceutical industry because of what has been proposed as poor cell permeability and lack of oral bioavailability. We have challenged this view by mining a comprehensive dataset of close to 500 drugs in bRo5 space, and by analyzing >200 macrocycles selected from the Broad Institute's DOS collection. Our investigations have shown that permeability and oral bioavailability can be achieved far outside Lipinski's rule of 5, in bRo5 space. Drugs in this space have the advantage of being able to modulate novel targets classes that often have difficult, open and featureless binding sites. We have also found that conformational flexibility, which may involve intramolecular interactions such as hydrogen bonding, improves cell permeability in bRo5 space.
We are continuing to analyze our databases and libraries to increase our understanding of the opportunities in bRo5 space and to find novel design strategies for this space. In particular, we are studying how intramolecular hydrogen bonding can be modulated and predicted to improve design of chemical probes and drugs. The knowledge generated in the project will be applied to design of compounds that modulate targets with difficult binding sites, with emphasis on epigenetic targets that regulate cancer cell growth. Our first publication in the field (J. Med. Chem., 2014, 57, 278−295) has been cited extensively and we have been invited to present our results at several conferences in the US, Europe and Japan.
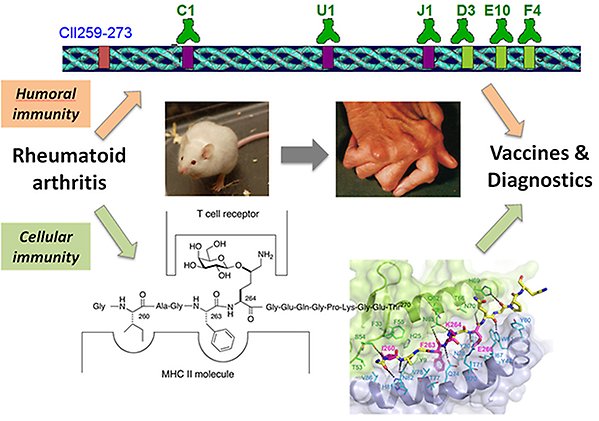
Development of vaccines and diagnostics for rheumatoid arthritis
Rheumatoid arthritis (RA) is a complex autoimmune disease that affects <1% of the population. We have found that the highly specific presentation of a glycopeptide derived from type II collagen to T cell receptors (TCRs) by class II MHC molecules is a key step in pathogenesis in a mouse model for RA. We also discovered that vaccination of mice with the glycopeptide bound to the MHCII molecule provided protection from disease. In humans RA is associated with an MHCII molecule that binds the glycopeptide differently than the mouse variant. We will now generate an understanding of the human system by investigating the molecular signaling that occurs in the MHCII – glycopeptide - TCR complex using sets of synthetic glycopeptide analogues. In addition to providing knowledge of molecular recognition between cells in the immune system the project will provide a basis for development of glycopeptide and glycoprotein based vaccines that halt the progression of RA, and for diagnostics of RA. This project has resulted in >25 joint publications and has positioned us as leaders in the field at the international level.
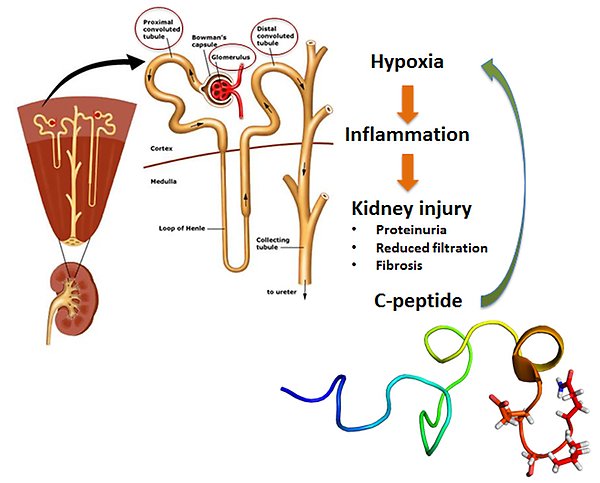
Chemical probes for studying chronic kidney disease
Chronic kidney disease (CKD) affects 10-16% of the world’s adult population, but lack of understanding of the complex pathways that drive the disease has hindered development of treatments. Hypoxia has been implicated as a driver for CKD and probes that can reduce hypoxia, such as C-peptide, therefore provide opportunities to increase our understanding of the pathogenesis and to find treatments. One aim of this project is to discover the targets of C-peptide and to determine the pathways through which it reduces hypoxia. Use of radiolabelled C-peptide has confirmed that it acts in the kidney and investigations at the cellular and subcellular level will now be conducted. In parallel an oxygen consumption assay in mouse tubuli cells is being developed and will be used to evaluate C-peptide mimetics in for reduction of hypoxia. Potent compounds will then be studied in a rat model for CKD with the long-term aim of finding novel and effective treatments for CKD.