Research projects in Pilarski Group
Our group invents and develops new methods to build organic molecules in ever faster, cheaper and more environmentally sustainable ways. We use several strategies to achieve this.
Catalytic C-H functionalisation
Catalysis has revolutionized organic synthesis. It enables molecules to be constructed in incredibly selective ways. We are particularly interested in developing and understanding transition metal-catalysed reactions that selectively replace C-H bonds with other functional groups; in principle one of the most efficient strategies for building up molecular complexity. It can greatly shorten the routes to valuable compounds and it can lead to transformations and products that are previously impossible.
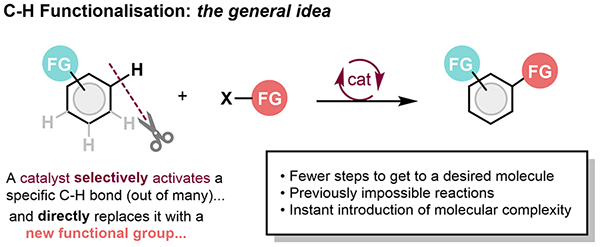
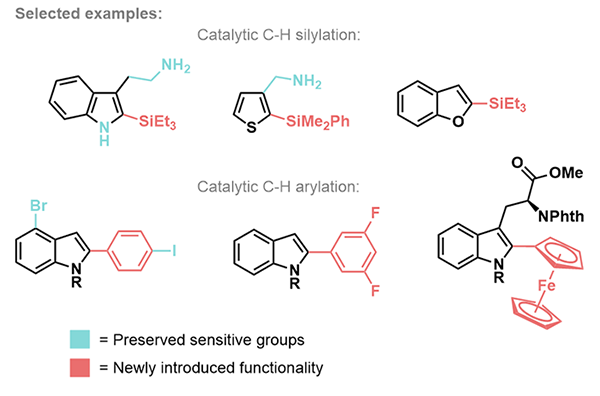
C-H bonds are the most prevalent type of “functional group” in organic molecules; getting C-H activation right means developing catalysts and strategies to change the desired C-H bond selectively (regioselectivity) for the right new group under the mildest possible conditions. Key to this is designing the catalyst correctly and understanding the mechanism by which these reactions occur. We are therefore also interested in aspects of organometallic chemistry (the chemistry of carbon-metal bonds). We have developed new C-H functionalisations of heterocycles that install C-C and C-Si bonds directly and preserve valuable functionality that would otherwise be destroyed but that is valuable in transformations further down the line.
Aryne chemistry
Arynes are short-lived, highly reactive intermediates with a formal “triple bond” in an aromatic ring. They have huge potential in synthesis because they can undergo a very large variety of valuable transformations. Both carbons of the triple bond can react in one step to produce very many different C-C and C-heteroatom bond combinations. Moreover, this can happen under exceptionally mild conditions. Arynes are, therefore, excellent candidates for versatile “building block” molecules from which many, very different new compounds may be constructed in a few steps.
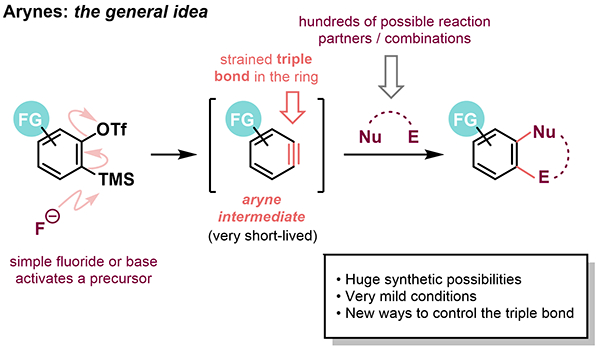
We are interested in generating the aryne triple bond in evermore complex and interesting contexts. We also study how to control the reactivity of the triple bond in new ways using groups that exert an influence from far away in the molecule. To achieve these goals, we use C-H activation to introduce new groups directly to various aryne precursors. We also study their properties, in part through mechanistic investigations and partly via collaborations we have established with computational chemists.
Mechanochemistry
Solvent accounts for a huge amount of the waste organic synthesis produces. For example, approximately 70% of the waste in pharmaceutical synthesis is attributable to solvent.
We are developing reactions that work under mechanochemical conditions, wherein molecules are forced to collide using physical force, sometimes entirely in the solid phase. This can, in principle, eliminate the need for solvent and therefore lead to huge advances in making synthesis greener and mores sustainable.
