Chemical and Bio-Molecular Physics
We address the fundamental properties and ultrafast processes in atoms, molecules, chemical systems, and proteins. With X-ray scattering and spectroscopy, we reveal how electrons and nuclei move and interact to explain how properties and function emerge.
Our research program addresses fundamental aspects in atomic, molecular, chemical, and biological systems. We develop and apply novel X-ray methods and instrumentation to reveal how transient electronic structures and nuclear dynamics determine properties and function. We use X-ray scattering and spectroscopy to understand and control the photophysics and photochemistry of atoms and molecules, aqueous solutions, metal complexes, and proteins. Our interdisciplinary research program is based on strong collaborations in experiment and theory and aims at applications in environmental, biophysical and the chemical sciences.
Research groups
- Light-matter interactions Hans Ågren
- Molecules and liquids Olle Björneholm
- Biophysics Carl Caleman and Nicusor Timneanu
- Atomic and molecular physics Johan Söderström and Jan-Erik Rubensson
- Chemical physics and dynamics Philippe Wernet
Division and partner program
We are in charge of the HELIOS lab for ultrafast soft X-ray spectroscopy and we belong to the X-ray Photon Science division together with the research program on Condensed Matter Physics on Energy Materials.
Light Matter Interactions
Light-matter interactions – Ågren group
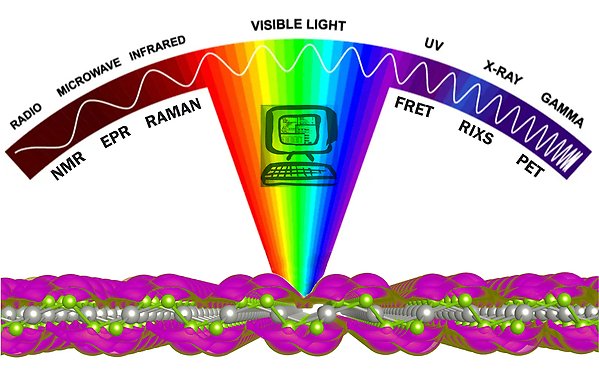
We are a group engaged in theory development and computational applications of light-matter interaction covering a wide part of the electromagnetic spectrum, from gamma-rays, where we optimize properties of positron emission tracers for early diagnosis of neurodegenerative diseases, over to spectroscopy in the X-ray range, to electromagnetic properties of 2D and plasmonic materials in the optical and UV regions, all the way down to the infrared (IR, Raman) and the radiofrequency regions (EPR, NMR).
We are currently working on five main topics:
- Resonant Inelastic X-ray Scattering
- 2D photodetector materials
- Biomarkers for neurodegenerative diseases
- Ultrafine nanoparticle plasmonics
- Light upconversion technology for energy harvesting applications.
Our research concerns theory, method development, computational science and problem oriented applications in collaboration with experimentalists.
Members of the group
Artem Kuklin, Junhao Li, Lucas Cornetta, Jingjian Zhou, Boris Minaev, Hans Ågren
Former members of the group (recent)
Molecules and Liquids
Molecules and liquids – Björneholm group
X-ray-induced dynamics of biomolecular model systems to understand the first steps of radiation damage
X-rays are widely used in e.g. medicine, both for diagnostics and for treatment (radiation therapy), as well as in science, e.g. in crystallography. In these applications, radiation damage is either a desired result or an unwanted side effect. On the molecular scale, radiation damage means breaking of bonds and fragmentation. With the aim of understanding the first steps of radiation damage, we explore X-ray-induced electron and nuclear dynamics in biologically relevant molecules and ions. Our approach to address these interconnected molecular-scale processes is to isolate different aspects by using selected types of model systems:
- Studies of gas-phase species to understand the intramolecular x-ray-induced electronic and nuclear dynamics on the low femtosecond timescale, in particular fragmentation without the influence of solvation.
- Studies of small ions in solution to understand the intermolecular charge transfer processes, such as intermolecular Coulombic decay (ICD) and Energy-transfer mediated decay (ETMD), with minimal influence of nuclear dynamics.
- Comparative studies of the same species in gas phase and in solution allow investigations on how the dynamics is modified by the surrounding water molecules.
Environmental molecular science
Atmospheric aerosols partially counteract the greenhouse effect by cooling the Earth via reflection of in-coming solar radiation and promoting cloud formation. According to the UN climate change panel IPCC, the magnitude of these effects is a major uncertainty in climate modelling, and it is crucial to constrain these better. The surface is important for aerosols due to their small size, but surface effects are not generally taken into account in current climate models. To learn about aerosol surfaces, we investigate how the surfaces of aqueous solutions of atmospherically relevant inorganic ions and organic molecules differ from the bulk in terms of composition, concentration and speciation.
Our tools for all these studies are various types of electron, x-ray and ion spectroscopies using x-rays from synchrotron radiation sources and free electron lasers, in combination with theoretical simulations.
Interested? Contact Olle Björneholm
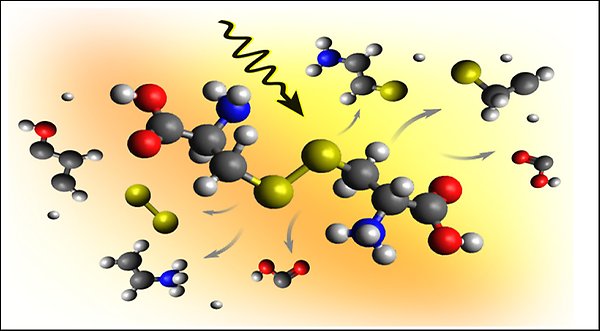
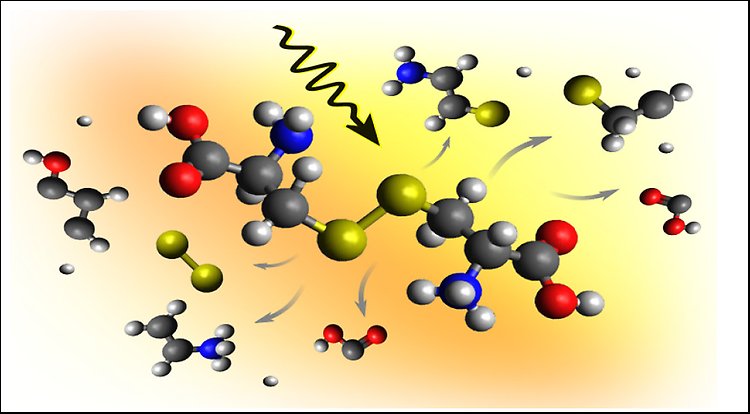
X-ray-induced breaking of a protein-like sulfur bridge in cystine.
Biophysics Biofysik Molecular Dynamics & Structure
A main part of our research revolves around X-ray free-electron lasers, which are novel light sources that provide extremely intense (1012 photons/pulse) and ultrashort (10-100 fs) X-ray pulses. These unique properties open new possibilities to determine structural information of biomolecules with atomic resolution, using a technique called diffraction before destruction. We are studying the ultrafast dynamics, fragmentation and non-thermal heating induced by X-ray free electron lasers.
We use Molecular Dynamics, Quantum Chemistry and Plasma Simulations to study ultrafast dynamics in biomolecules. We are interested in the atomic dynamics and study how to improve single particle imaging using information from fragmentation and orientation. We are working on model development in photon-matter interaction at high X-ray field strengths, complemented with performing experiments at X-ray facilities.
We are one of the founders of the Biophysics Network at Uppsala University and the Master’s Programme in Biophysics.
Members of the group
Carl Caleman, Nicusor Timneanu, Ibrahim Dawod, Thomas Mandl, Sebastian Cardoch, Pamela Svensson, Gösta Huldt, Tomoko Akiyama, Tomas André
Former members of the group (recent)
Isaak Unger, Kajwal Patra (now at U. Dallas), Emiliano de Santis (now at BMC), Christofer Östlin, Clara Saak (now at U. Wien), Olof Jönsson (now at Cytiva), Davide Ragazzon
same?
Mol D-Struct – Molecular Dynamics & Structure
We are a group within the Division of X-ray Photon Science that focuses on research of structure and dynamics of molecular systems. We mainly use X-rays from synchrotrons as well as X-ray Free-Electron Lasers to investigate matter at an atomic level.
The group consists of researchers of varying backgrounds involved in a number of different projects. To gain further insight into our research you may read through some of our published articles. We also offer various student projects in our group.
We are part of the Biophysics Initiative at the Department of Physics and Astronomy, and the Biophysics Network at the Faculty of Science and Technology.

People
The group currently consists of the following people:
Carl Caleman
PhD in Molecular Biophysics 2007. Docent in Physics 2018.
Nicusor Timneanu
PhD in Theoretical Particle Physics 2002, Docent in Molecular Biophysics 2009.
Isaak Unger (post doc)
Kajwal Patra (post doc)
Emiliano de Santis (post doc)
Sebastian Cardoch (PhD student)
Ibrahim Dawod (PhD student)
Pamela Svenson (PhD student)
Thomas Mandl (PhD student)
Previous members
Christofer Östlin – PhD 2019
Clara Saak – PhD 2019
Olof Jönsson – PhD 2017
Davide Ragazzon – postdoc
Research
A main part of our research revolves around X-ray free-electron lasers, which are novel light sources that provide extremely intense (1012 photons/pulse) and ultrashort (10-100 fs) X-ray pulses. These unique properties open new possibilities to determine structural information of biomolecules with atomic resolution, using a technique called diffraction before destruction. We are studying radiation damage and non-thermal heating induced by X-ray free electron lasers in biological samples.
We use Molecular Dynamics simulations and Quantum Chemistry to study ultrafast electron dynamics in biomolecules. We are also interested in the atomic dynamics, and study the orientation of molecules using laser fields, or orientation in-silico of biomolecules after being exposed to an X-ray pulse. Furthermore, we are working on force field development in molecular dynamics.
In close collaboration with experiments, we investigate liquid surfaces using synchrotron radiation as well as Molecular Dynamics simulations. We study the solvation properties of inorganic salts close to the water/vapor interface.
Bachelor and master projects
We offer students at both bachelor and master levels the possibility to carry out their thesis work with us. This is an excellent opportunity to establish academic contacts while also gaining insight into the world of research. Below is a list of current project proposals. Also have a look at our published master theses.
¤ Molecular dynamics simulations of protein molecules in laser fields
Simulation study of how the native atomic structure of a protein is affected as it is exposed to a laserfield. Lasers are used as optical tweezers and this study aims to understand how the electric field, the laser field, actually affects the protein structure. The project will involve learning how to use the molecular dynamics program GROMACS.
¤ Validating water models for molecular modeling
In molecular modeling water is often present in one way or another. There are over 50 different water models used by scientists when modeling different phenomena. This project is about comparing the physical and chemical properties of a subset of all the available models to decide which models that are good at what. The project will involve learning how to use the molecular dynamics program GROMACS and learning how to evaluate simulations.
¤ Molecular dynamics of organic molecules on water surfaces
The behavior of small organic molecules on water surfaces is important for atmospheric chemistry. Molecules that show surface preference have a larger possibility to interact with the surrounding atmosphere. We have studied how small organic molecules such as carboxylic acids and alcohols behave in a water/gas interphase both experimentally and using molecular dynamics. This project is focused on doing a simulation study of how the structure of different organic molecules affect the molecules' surface preference. Simulations will be done using the molecular dynamics package GROMACS and will be strongly connected to experimental results that come from studies at synchrotron sources such as MAXlab.
¤ Shockwaves in materials induced by an X-ray laser
X-ray lasers are new types of lasers, which produce extremely intense and short X-ray pulses. In this project you will use computer simulations to study how shockwaves can be created in a material (e.g. metal) when it is hit by a focused laser beam and turns into a plasma. This will help us understand how the structure of the material changes and how to control such an extreme process.
¤ Experimental Study and Simulation of Liquid Interfaces
The properties of liquid surfaces and interfaces differ fundamentally from the bulk. It is therefore crucial to understand the behaviour of solutions in proximity to such an interface. Two powerful tools to study these systems are photoelectron spectroscopy and molecular dynamics (MD) simulations. In this project you would study aqueous solutions containing various solutes (e.g. small molecules and ions) using our in-house experiment as well as synchrotron light source setups. Our setup combines a liquid jet with a hemispherical photoelectron analyzer, which allows us to selectively observe the interface of the liquid and can also be modified to study aerosols. The experimental results would then be supported by MD simulations which are crucial if one wants to understand the mechanisms behind the observed experimental effect.
Aerosols
Fundamental Environmental Molecular Science
Earth seen from space is dominated by blue, white and green areas, which are associated with various aqueous systems of the biosphere. These consist of condensed water with various dissolved species and interacting with molecules, solid surfaces and solar radiation. Environmental molecular science aims to increase our fundamental, molecular-level understanding of natural and anthropogenic processes in the biosphere. This understanding may be used to guide efforts to prevent and reduce human-induced environmental problems.
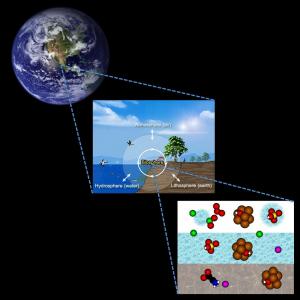
We carry out fundamental experimental research on the chemical physics of liquids and nanoparticles, studying carefully designed model systems to understand natural and anthropogenic processes in the biosphere. Below you can get a flavour of the whys, whats and hows of our research:
- Aqueous surfaces and atmospheric science
- Ions at aqueous surfaces
- Molecules at aqueous surfaces
Aqueous surfaces and atmospheric science
Atmospheric aerosols partially counteract the greenhouse effect by cooling the Earth via reflection of in-coming solar radiation (the direct effect), and promoting cloud formation (the indirect effect). According to the UN climate change panel IPCC, the magnitude of these effects is the major uncertainty in climate change prediction, and it is crucial to constrain these better to improve climate modeling. The surface is important for aerosols due to their small size, but surface effects are not generally taken into account in current climate models.
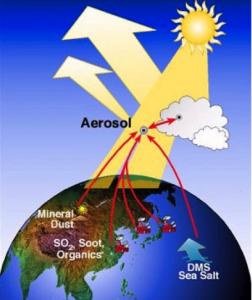
In the atmosphere there are species of both natural and anthropogenic origin incorporated into aqueous aerosols. This includes salt ions from the sea, organic molecules from both direct emissions and decomposition of biomaterial, soot from combustion, pollutants and mineral particles.
We investigate how the surfaces of aqueous solutions differ from the bulk in terms of composition, concentration and speciation. We do this in close collaboration with atmospheric scientists, such as Ilona Riipinen, Nønne Prisle and Joakim Pagels.
Ions at aqueous surfaces
The surface of aqueous systems is different from the bulk. For ions in macroscopic liquid water, our understanding is now changing from a model with an ion-free surface to a model in which ions may be present, and even enriched, at the surface.
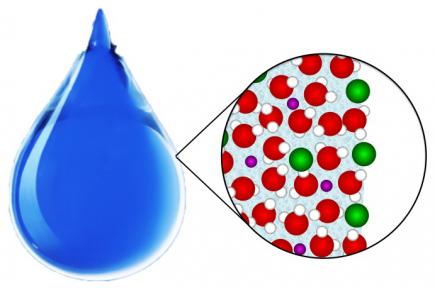
Ions enriched or depleted at the aqueous surface
In nature such phenomena are for example important when droplets of sea spray form aerosol particles in the atmosphere. The sea spray droplets contain ions, and as water gradually evaporates, the droplets become smaller and concentration increases. The resulting aerosol particles are involved in atmospheric chemistry, and act as nucleation centers for cloud formation.
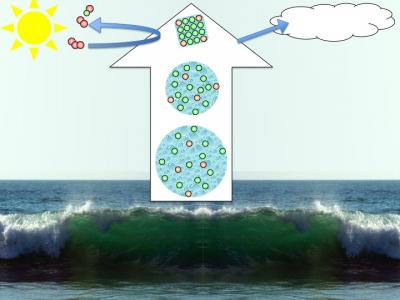
From sea-spray to saline aerosol particles
We have studied how the surface composition of mixed seawater-like solutions subjected changes with concentration. The minority bromide ions become increasingly more abundant at the surface relative to the majority chloride ions with increasing concentration, which could explain why bromine is much more important in atmospheric chemistry than expected from its relative abundance in seawater (PCCP 12, 10693 (2010)).
In the atmosphere, the ions of marine origin undergo chemistry. We have studied the oxychlorine ions (ClOx–, x=0–4), which e.g. are formed by stepwise oxidation of Cl– by ozone. The increasing intensities observed in XPS, tells us how the surface propensity increases from Cl– to ClO4– (JCP 131, 124706 (2009)).
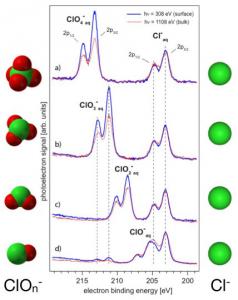
Cl2p XPS spectra of oxychlorine ions, revealing varying surface propensity
Molecules at aqueous surfaces
In the atmosphere, primary hydrocarbons are gradually converted into a plethora of partially oxidized organic molecules, for example alcohols and carboxylic acids.
Alcohols
We have studied the behavior of alcohols such as pentanol on the water surface. Combining XPS and MD, we show that the pentanol molecules are strongly surface enriched (PCCP 17, 14036 (2015)). At low concentrations, the molecules are seen to “lie down” on the surface, and gradually “stand up” with increasing concentration to make room for more molecules.
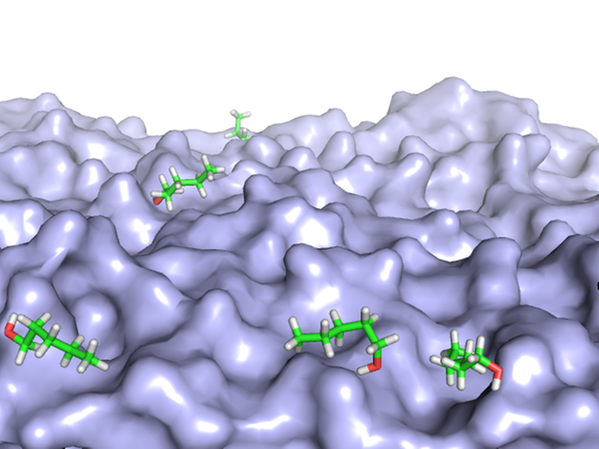
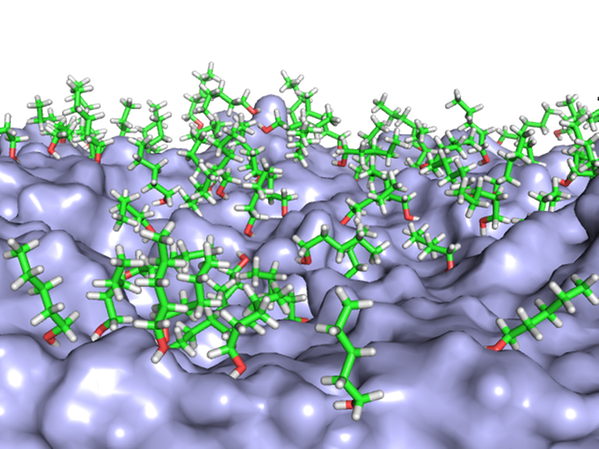
MD snapshots of pentanol molecules on the water surface at low and high concentration.
Carboxylic acids
Carboxylic acids, formed by e.g. oxidation of hydrocarbons, are important compounds in atmospheric chemistry. For carboxylic acids and their de-protonated, carboxylate, conjugate base forms in aqueous solution, we have shown that the acid forms have a higher surface propensity than the corresponding carboxylates, here schematically exemplified for acetic acid (PCCP 13, 12261 (2011)).
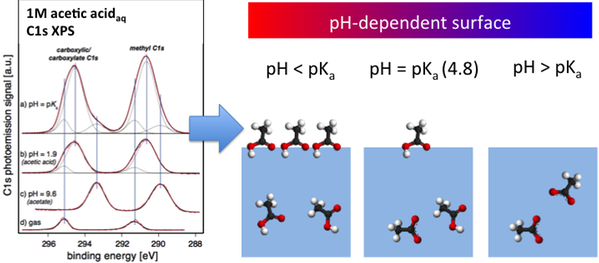
The surface composition is seen to differ from the bulk at all pH, both in terms of speciation and concentration.
Succinic acid
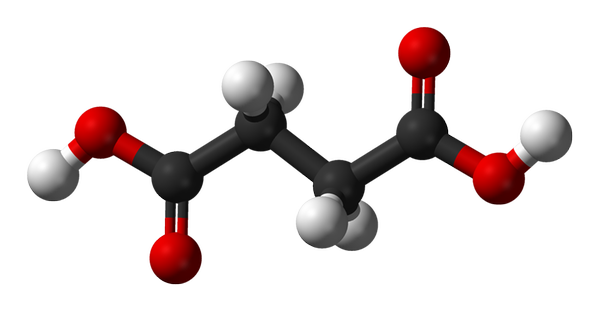
Succinic acid is often used as a representative of dicarboxylic acids. Using XPS and MD, we have shown how the surface propensity of succinic acid varies with pH (PCCP 16, 21486 (2014)). While the deprotonated species Succ2+ essentially avoids the surface, the neutral protonated species SuccH2 is surface enriched. XPS allows quantitative determination of the surface composition as function of bulk concentration.
Solutes affect the surface tension of water, which is a key parameter in cloud droplet formation, as described by Köhler theory.

Schematic illustration of the growth from cloud condensation nuclei (CCN) to large cloud droplets.
We have derived the surface tension from the surface composition determined by XPS of succinic acid solutions.
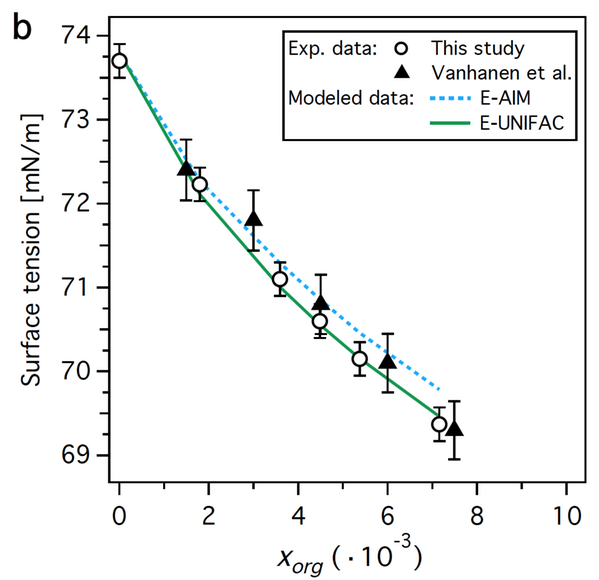
Surface tension as function of succinic acid mole fraction.
We can thus understand the microscopic origins of macroscopic properties such as surface tension, which is a step towards improving the description of aerosol effects in atmospheric models.
Surface-specific proton-transfer reactions
For longer carboxylic, “fatty”, acids, we studied the effects of some of the most common atmospheric inorganic ions, Na+, NH4+, Cl−, and SO42− (ACPD 12, 12227 (2012), JPCB 119, 4033 (2015)). Only NH4+ was seen to have an effect, namely a pronounced surface enhancement of “fatty” acid, which is attributed to surface-specific acid–base chemistry.
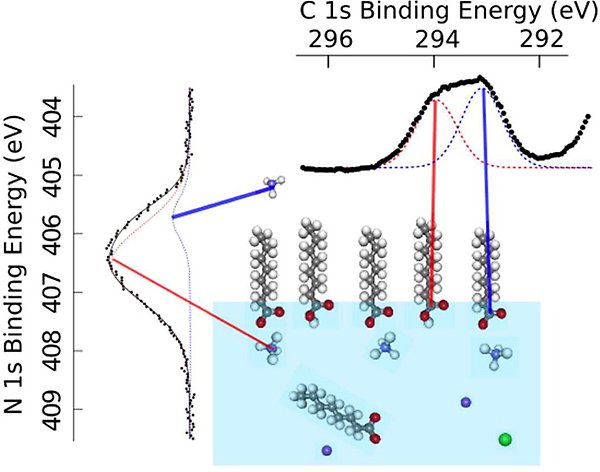
Schematic illustration of surface-specific acid–base chemistry between octanoate and ammonium, leading to the formation of octanoic acid on the surface and the release of ammonia, NH3, into the gas phase.
Future
In the future, we will extend these studies to more realistic model systems of atmospheric systems. This will include aerosols produced by atomizers and sea spray simulation tanks.
Contact
Who are we?
- Olle Björneholm, professor
- Gunnar Öhrwall, researcher (MAX-lab, Lund)
- Carl Caleman, researcher
- Josephina Werner, researcher
- Isaak Unger, post-doctoral researcher
- Victor Ekholm, PhD student
- Clara Saak, PhD student
Alumni
- Wandared Pokapanich, Nakhon Phanom University, Thailand
- Niklas Ottosson, AMOLF, Amsterdam, the Netherlands
Please contact us if you are interested in our research! We can offer diploma projects (ex-jobb).
Clusters
Clusters - From the Isolated Atom to the Infinite Solid
Imagine that you take a piece of an ordinary material, let's say a bar of gold. If you divide this in two halves, the two parts will still be gold metal, i.e. they will have the same physical properties. Can you continue this division of the gold metal infinitely and still have small pieces of metallic gold?
The answer to that question is a part of cluster physics. Clusters are aggregates of a small and finite number of atoms or molecules. They range from the dimer, consisting of only two atoms, up to large clusters made up of several tens of thousands of atoms. In this sense they bridge the gap between the isolated atom and the infinite solid. By following the development of various physical and chemical properties from the atom, over clusters of increasing size to the infinite solid, a better understanding of the microscopic origin of macroscopic properties may be gained.
Because of their small sizes, the surface, i.e. the outermost layer of atoms, of the clusters is very important for their properties. Atoms at the surface of the cluster are located in sites with a reduced number of nearest neighbours. This results in size-dependent changes in electrical, magnetic and optical properties, chemical reactivity and catalytic activity. In addition to this, the small number of atoms result in quantum-size effects. For small cluster sizes, these variations are very strong and not just a simple linear function of size. For larger clusters, the fluctuations with size get gradually weaker, and there is a smooth development of the properties towards those of the infinite solid. This size-dependence of chemical and physical properties is the subject of intense research, with the aim of tuning the properties to desired values by selection of clusters of suitable size, which forms the basis for many of the proposed practical applications of clusters. These include the use of clusters deposited on surfaces for heterogeneous catalysis, or the construction of novel materials, where the building blocks are rather clusters than individual atoms.
From a basic scientific viewpoint it is highly desirable to obtain a more detailed understanding of the general development of the electronic and geometric structure of clusters. Furthermore, an improved understanding of free clusters is also essential for research in more directly applied fields. We have studied both rare gas and molecular clusters, and we are now focusing on clusters of metals and semiconductors. For these we have developed in vacuo synthesis methods, allowing us to both produce very pure clusters with controlled composition and radial structure, as exemplified below for Yb/YbO.
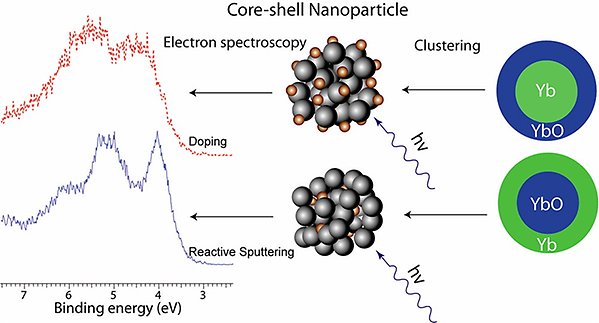
Core-shell nanoparticles of Yb and its divalent oxide YbO have been created by two different approaches, resulting in either Yb in the core and YbO in the shell or vice versa [J. Phys. Chem. C 117, 14390 (2013)].
Researchers
- Olle Björneholm
- Maxim Tchaplyguine (MAX-lab, Lund)
Water
Charge Delocalization Dynamics in Aqueous Systems
The interaction between water molecules and solutes, both ions and molecules, is fundamental to the understanding of all aqueous systems, but our understanding of the wide range of complex interactions is far from complete. One aspect of this is to what degree electrons on neighboring molecules will participate in electronic processes. For the Auger process, in which a hole in a core orbital within a few femtoseconds is filled by a valence electron while another valence electron is ejected, there are very few inter-molecular processes for weakly bonded van der Waals systems, whereas “inter-molecular” processes dominate for more strongly bonded covalent and metallic systems. We study what the situation is for aqueous systems, in which the strength of the hydrogen bonding is intermediate to the weak and strong cases.
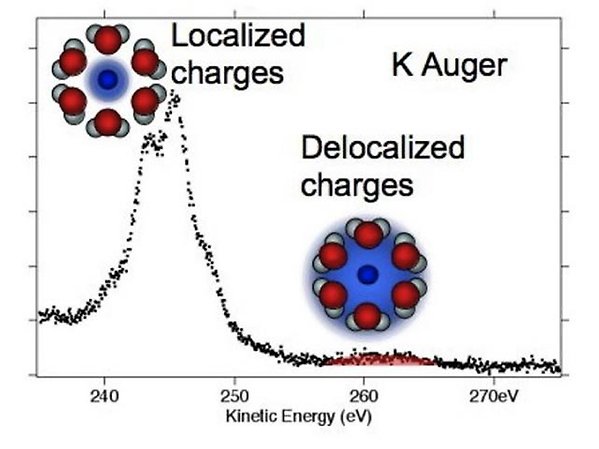
K2p Auger spectra of aqueous potassium ions, revealing ICD-like decay processes leading to delocalized charges.
We have found that for aqueous solutions of salts, i.e. KCl, the overlap of the water molecules surrounding the ions in the solution is such that electrons from the water molecules take part in the electronic relaxation after core ionization for the potassium ion, but not for the chloride ion (JACS 131, 7264 (2009)). Naively one would expect only the electrons on the potassium ion to take part, as the core hole created by photoionization is strongly localized, but we find clear evidence that to some degree delocalized electrons from water molecules must be involved in an ICD-like process.
We have shown several examples of such ultrafast charge-delocalization dynamics in aqueous electrolytes revealed by Auger spectroscopy. This includes phenomena such as ICD-like decay processes and electron delocalization associated to both anionic and cationic species JPCB 114, 17057 (2010), JACS 133, 13430 (2011)].
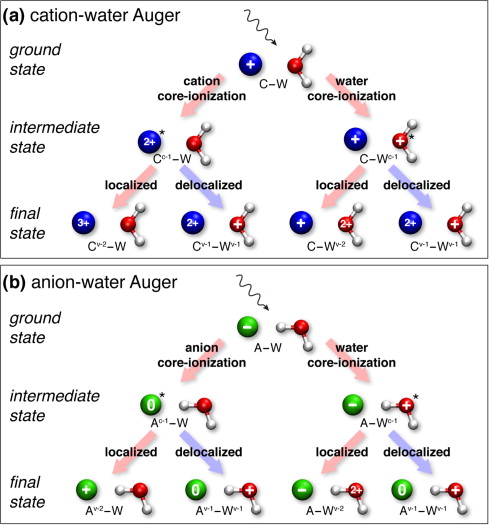
Schematic flow diagram of initial, intermediate and final state in the Auger process following absorption of ionizing X-ray radiation in aqueous electrolytes.
We have also seen related processes for the solvating water molecules. The efficiency is affected by solutes such as anions and cations. Using the core–hole-clock, we can obtain quantitative results, which reveal timescales ranging from less than one to hundreds of femtoseconds [JACS 133, 13489 (2011) JPCB 116, 3 (2012)].
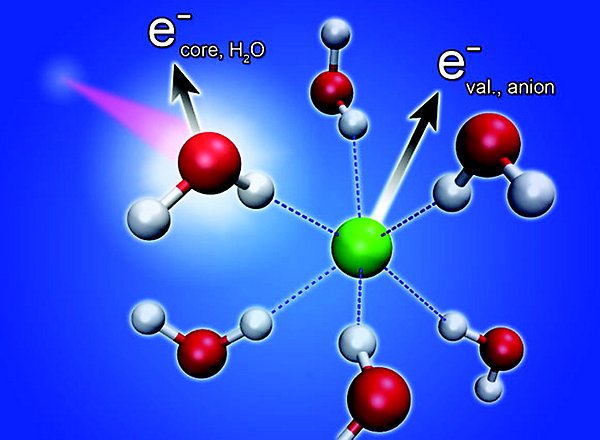
Schematic illustration of a non-local decay channel for core-ionized water in an anion solvation shell.
On a qualitative level, the observed differences in dynamics can be understood by relatively simple models of the water-ion interaction [CPL 543, 1 (2012)].
Future
We will extend these studies to Auger cascades after deep core ionization, and to truly time-resolved studies using the HELIOS setup for pump-probe electron spectroscopy.
Who are we?
- Olle Björneholm, professor
- Gunnar Öhrwall, researcher (MAX-lab, Lund)
- Carl Caleman, researcher
- Josephina Werner, researcher
- Isaak Unger, post-doctoral researcher
- Victor Ekholm, PhD student
- Clara Saak, PhD student
Alumni
- Wandared Pokapanich, Nakhon Phanom University, Thailand
- Niklas Ottosson, AMOLF, Amsterdam, the Netherlands
Please contact us if you are interested in our research! We can offer diploma projects (ex-jobb).
Contact
- Programme Professor Chemical and Bio-Molecular Physics
- Philippe Wernet
