Water
Charge Delocalization Dynamics in Aqueous Systems
The interaction between water molecules and solutes, both ions and molecules, is fundamental to the understanding of all aqueous systems, but our understanding of the wide range of complex interactions is far from complete. One aspect of this is to what degree electrons on neighboring molecules will participate in electronic processes. For the Auger process, in which a hole in a core orbital within a few femtoseconds is filled by a valence electron while another valence electron is ejected, there are very few inter-molecular processes for weakly bonded van der Waals systems, whereas “inter-molecular” processes dominate for more strongly bonded covalent and metallic systems. We study what the situation is for aqueous systems, in which the strength of the hydrogen bonding is intermediate to the weak and strong cases.
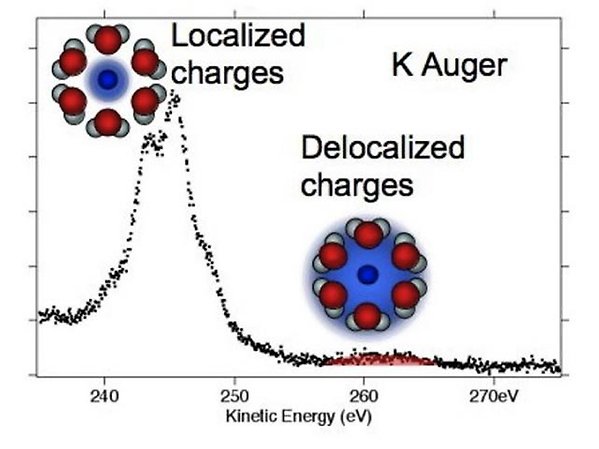
K2p Auger spectra of aqueous potassium ions, revealing ICD-like decay processes leading to delocalized charges.
We have found that for aqueous solutions of salts, i.e. KCl, the overlap of the water molecules surrounding the ions in the solution is such that electrons from the water molecules take part in the electronic relaxation after core ionization for the potassium ion, but not for the chloride ion (JACS 131, 7264 (2009)). Naively one would expect only the electrons on the potassium ion to take part, as the core hole created by photoionization is strongly localized, but we find clear evidence that to some degree delocalized electrons from water molecules must be involved in an ICD-like process.
We have shown several examples of such ultrafast charge-delocalization dynamics in aqueous electrolytes revealed by Auger spectroscopy. This includes phenomena such as ICD-like decay processes and electron delocalization associated to both anionic and cationic species JPCB 114, 17057 (2010), JACS 133, 13430 (2011)].
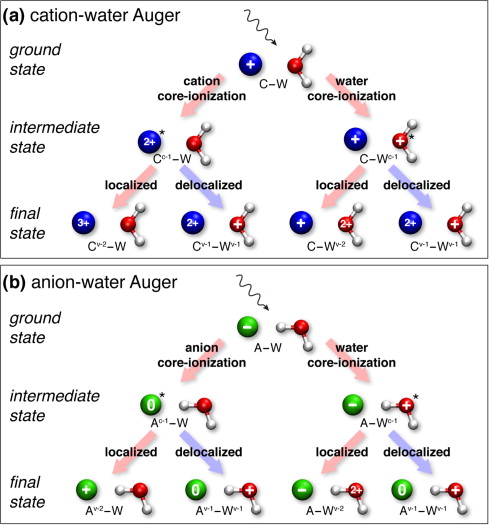
Schematic flow diagram of initial, intermediate and final state in the Auger process following absorption of ionizing X-ray radiation in aqueous electrolytes.
We have also seen related processes for the solvating water molecules. The efficiency is affected by solutes such as anions and cations. Using the core–hole-clock, we can obtain quantitative results, which reveal timescales ranging from less than one to hundreds of femtoseconds [JACS 133, 13489 (2011) JPCB 116, 3 (2012)].
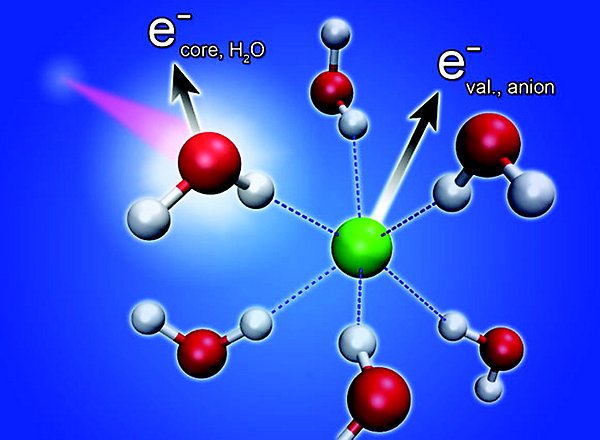
Schematic illustration of a non-local decay channel for core-ionized water in an anion solvation shell.
On a qualitative level, the observed differences in dynamics can be understood by relatively simple models of the water-ion interaction [CPL 543, 1 (2012)].
Future
We will extend these studies to Auger cascades after deep core ionization, and to truly time-resolved studies using the HELIOS setup for pump-probe electron spectroscopy.
Who are we?
- Olle Björneholm, professor
- Gunnar Öhrwall, researcher (MAX-lab, Lund)
- Carl Caleman, researcher
- Josephina Werner, researcher
- Isaak Unger, post-doctoral researcher
- Victor Ekholm, PhD student
- Clara Saak, PhD student
Alumni
- Wandared Pokapanich, Nakhon Phanom University, Thailand
- Niklas Ottosson, AMOLF, Amsterdam, the Netherlands
Please contact us if you are interested in our research! We can offer diploma projects (ex-jobb).
Contact
- Programme Professor Chemical and Bio-Molecular Physics
- Philippe Wernet
