Clusters
Clusters - From the Isolated Atom to the Infinite Solid
Imagine that you take a piece of an ordinary material, let's say a bar of gold. If you divide this in two halves, the two parts will still be gold metal, i.e. they will have the same physical properties. Can you continue this division of the gold metal infinitely and still have small pieces of metallic gold?
The answer to that question is a part of cluster physics. Clusters are aggregates of a small and finite number of atoms or molecules. They range from the dimer, consisting of only two atoms, up to large clusters made up of several tens of thousands of atoms. In this sense they bridge the gap between the isolated atom and the infinite solid. By following the development of various physical and chemical properties from the atom, over clusters of increasing size to the infinite solid, a better understanding of the microscopic origin of macroscopic properties may be gained.
Because of their small sizes, the surface, i.e. the outermost layer of atoms, of the clusters is very important for their properties. Atoms at the surface of the cluster are located in sites with a reduced number of nearest neighbours. This results in size-dependent changes in electrical, magnetic and optical properties, chemical reactivity and catalytic activity. In addition to this, the small number of atoms result in quantum-size effects. For small cluster sizes, these variations are very strong and not just a simple linear function of size. For larger clusters, the fluctuations with size get gradually weaker, and there is a smooth development of the properties towards those of the infinite solid. This size-dependence of chemical and physical properties is the subject of intense research, with the aim of tuning the properties to desired values by selection of clusters of suitable size, which forms the basis for many of the proposed practical applications of clusters. These include the use of clusters deposited on surfaces for heterogeneous catalysis, or the construction of novel materials, where the building blocks are rather clusters than individual atoms.
From a basic scientific viewpoint it is highly desirable to obtain a more detailed understanding of the general development of the electronic and geometric structure of clusters. Furthermore, an improved understanding of free clusters is also essential for research in more directly applied fields. We have studied both rare gas and molecular clusters, and we are now focusing on clusters of metals and semiconductors. For these we have developed in vacuo synthesis methods, allowing us to both produce very pure clusters with controlled composition and radial structure, as exemplified below for Yb/YbO.
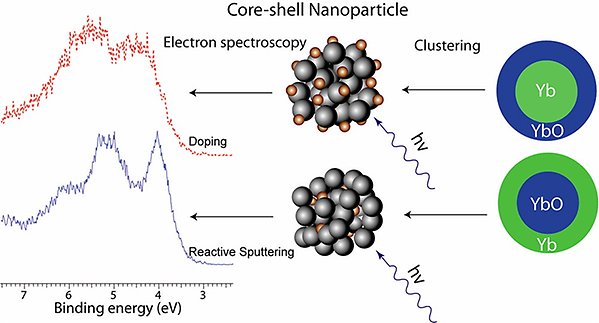
Core-shell nanoparticles of Yb and its divalent oxide YbO have been created by two different approaches, resulting in either Yb in the core and YbO in the shell or vice versa [J. Phys. Chem. C 117, 14390 (2013)].
Researchers
- Olle Björneholm
- Maxim Tchaplyguine (MAX-lab, Lund)
Contact
- Programme Professor Chemical and Bio-Molecular Physics
- Philippe Wernet
