Photovoltaics
Photovoltaics
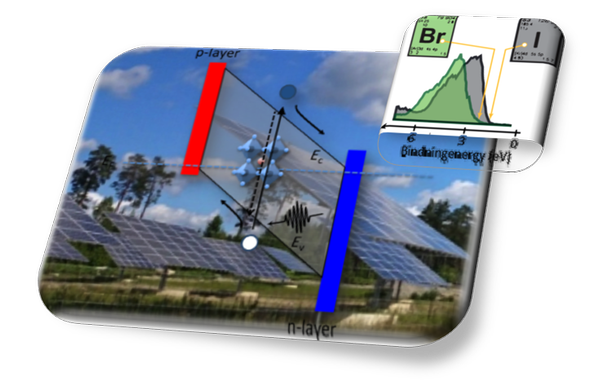
With the help of solar cells, solar energy can be directly converted to electrical energy with minimal environmental impact. Common to all solar structures is an interface layer where the sunlight is absorbed by exciting electrons (e-) and where the electrons are then rapidly transported from their positive environment, e.g. hole (h+). Through this process, the electrons increase their potential energy and create a photovoltage (U) which can then be used for an electrical current (I) and taken out as electrical energy with the power P = U x I.
The research focuses on understanding the formation and character of electrons and holes as well as how the charge separation takes place at the atomic level. From this basic understanding, we learn how to build new materials and interfaces that effectively and with high stability can be used to make the conversion from the sun's light to electricity as efficient and sustainable as possible.
Our activity focuses on the investigation of emerging solar cell technologies such as Perovskite Solar Cells (PSC), Quantum Dots Cells or Molecular Solar Cells (DSC and Polymer solar cells). The research focuses also on understanding how the formation of electrons and holes as well as the charge separation takes place at the atomic level. From this basic understanding, we learn how we can build new and more stable materials and interfaces that can be used efficiently in a solar cell device.
The understanding is obtained by using the characteristics of the X-ray spectroscopy in terms of element specificity and chemical sensitivity and we are constantly developing these methods of experimenting on functional systems to follow the transformation in time and space. In short, the X-ray spectroscopy is used to investigate where the absorption process occurs, where are the electrons, what time does it take for the charges to be transported from atom to atom, and loss-mechanisms including conversion of electrical energy to heat.
People
Håkan Rensmo (professor)
Sergey Butorin (researcher)
Bertrand Philippe (researcher)
Gabriel Man (Postdoc)
Dibya Phuyal (PhD student)
Sebastian Svanström (PhD student)
Selected publications:
Valence Level Character in a Mixed Perovskite Material and Determination of the Valence Band Maximum from Photoelectron Spectroscopy: Variation with Photon Energy
Bertrand Philippe, T. Jesper Jacobsson, Juan-Pablo Correa-Baena, Naresh K. Jena, Amitava Banerjee, Sudip Chakraborty, Ute B. Cappel, Rajeev Ahuja, Anders Hagfeldt, Michael Odelius, Håkan Rensmo.
J. Phys. Chem. C, 2017, DOI: 10.1021/acs.jpcc.7b08948.
Partially Reversible Photoinduced Chemical Changes in a Mixed-Ion Perovskite Material for Solar Cells
Ute B. Cappel, Sebastian Svanström, Valeria Lanzilotto, Fredrik O. L. Johansson, Kerttu Aitola, Bertrand Philippe, Erika Giangrisostomi, Ruslan Ovsyannikov, Torsten Leitner, Alexander Föhlisch, Svante Svensson, Nils Mårtensson, Gerrit Boschloo, Andreas Lindblad, Håkan Rensmo.
ACS Appl. Mater. Interfaces, 2017, 9, 34970-34978.
Chemical Distribution of Multiple Cation (Rb+, Cs+, MA+, and FA+) Perovskite Materials by Photoelectron Spectroscopy
Bertrand Philippe, Michael Saliba, Juan-Pablo Correa-Baena, Ute B. Cappel, Silver-Hamill Turren-Cruz, Michael Grätzel, Anders Hagfeldt, Håkan Rensmo.
Chem. Mater, 2017, 29, 3589-3596.
In-Situ Probing of H2O Effects on a Ru-Complex Adsorbed on TiO2 Using Ambient Pressure Photoelectron Spectroscopy
Eriksson SK; Hahlin M; Axnanda S; Crumlin E; Wilks R; Odelius M; Eriksson AIK; Liu Z; Ahlund J; Hagfeldt A; Starr DE; Bar M; Rensmo H; Siegbahn H.
Top Catal. 2016;59(5-7):583-90.
Electronic Structure of TiO2/CH3NH3Pbl3 Perovskite Solar Cell Interfaces
Lindblad R; Bi DQ; Park BW; Oscarsson J; Gorgoi M; Siegbahn H; Odelius M; Johansson EMJ; Rensmo H.
J Phys Chem Lett. 2014;5(4):648-53.
Atomic and Electronic Structures of Interfaces in Dye-Sensitized, Nanostructured Solar Cells
Johansson EMJ; Lindblad R; Siegbahn H; Hagfeldt A; Rensmo H.
ChemPhysChem. 2014;15(6):1006-17.
Research links
We are members of STandUP for energy and the Center for Molecular devices (CMD). The research platforms include strong links to Department of Chemistry Ångström and Department of Engineering at Uppsala University as well as groups doing similar research at KTH and Stockholm University. It consists of monthly meetings with 30-40 researchers and a large set of shared grants (e.g. SSF, STEM, VR), PhD students, postdocs and researchers as well as publications. Internationally our collaborations include University of Cambridge, United Kingdom and École Polytechnique Fédérale de Lausanne (EPFL), Switzerland.
Master and Bachelor projects
We invite students interested in master and bachelor projects. For more details about available projects, please contact Håkan Rensmo.
Contact
- Programme Professor Condensed Matter Physics of Energy Materials
- Håkan Rensmo
- Head of Division X-ray Photon Sciences
- Nicusor Timneanu
