Biocompatible ‘click-type’ reactions under physiological conditions
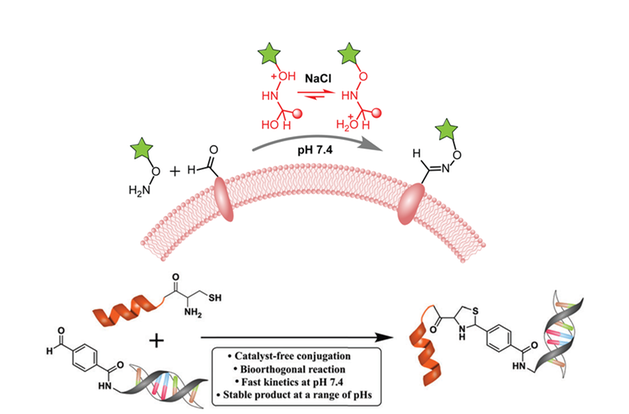
Reactions that yield stable ‘click-type’ coupling products could be used to develop drug conjugates.
Our project
There are several challenges to perform covalent coupling reactions under physiological conditions as the aqueous reaction condition limits the reactivity of nucleophilic and electrophilic reagents. We have discovered a simple and versatile condition to catalyze reactions to yield hydrazone, oxime and thiazolidine bonds. Such reactions are chemoselective and best suited for bioconjugation reactions with sensitive molecules such as DNA, RNA, peptide as well on the cell surface proteoglycans. Such reactions yield stable ‘click-type’ coupling products and could be used to develop drug conjugates.
Figure above: Novel covalent coupling strategies under physiological conditions.
Publications
- Wang, S.; Nawale, G. N.; Kadekar, S.; Oommen, O. P.; Jena, N. K.; Chakraborty, S.; Hilborn, J.; Varghese, O. P. Saline Accelerates Oxime Reaction with Aldehyde and Keto Substrates at Physiological pH. Sci. Rep. 2018, 8, 2193. https://doi.org/10.1038/s41598-018-20735-0
- Bermejo-Velasco, D.; Nawale, G. N.; Oommen, O. P.; Hilborn, J.; Varghese, O. P. Thiazolidine chemistry revisited: a fast, efficient and stable click-type reaction at physiological pH. Chem. Commun. 2018, 54, 12507-12510. http://dx.doi.org/10.1039/C8CC05405C
- Wang, S.; Gurav, D.; Oommen, O. P.; Varghese, O. P. Insights into the Mechanism and Catalysis of Oxime Coupling Chemistry at Physiological pH. Chem. Eur. J. 2015, 21, 5980-5985. https://onlinelibrary.wiley.com/doi/abs/10.1002/chem.201406458
