Cobalt-based catalysts for light-driven water oxidation
Our project
(This project is in collaboration with Sascha Ott and Stenbjörn Styring.)
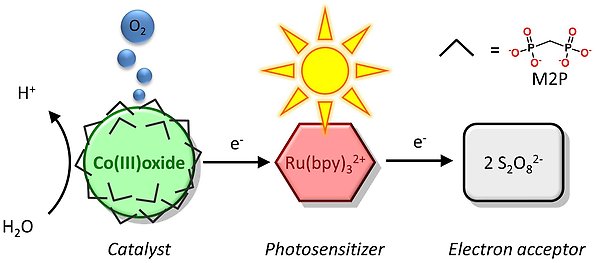
We have developed a number of oxygen evolving water oxidation catalysts that can be driven by visible light in aqueous solutions at neutral or weakly basic conditions. One of the catalysts is a heterogeneous catalyst based on amorphous cobalt oxide nanoparticles and uses methylenediphosphonate (M2P) as an additional ligand. The ruthenium complex Ru(bpy)32+ is used as the photosensitizer and peroxosulfate (S2O82-) as the sacrificial electron acceptor (Scheme below). The presence of a ligand allows for an anchoring point where photosensitizer and catalyst can be linked together.
Figure 1, above: Schematic representation of the light-driven water oxidation using cobalt oxide nanoparticles.
We have linked the photosensitizer to the cobalt catalyst by using a modified photosensitizer containing M2P groups, creating a hybrid Ru-Co molecular/heterogeneous material. We are currently modifying the photosensitizer to be able to attach to a TiO2 surface to get a complete photo-anode.
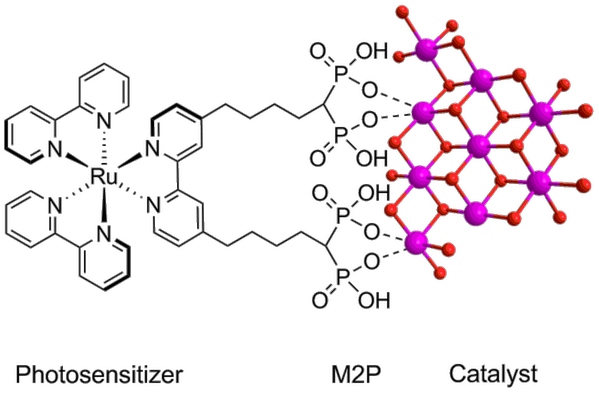
Figure 2. The Ru-Co material with a molecular photosensitizer linked to a heterogeneous water oxidation catalyst via coordination bonds.
We are also investigating molecular cobalt-based catalysts for water oxidation. We have been studying both mononuclear and dinuclear complexes with polypyridine ligands. The point of interest is the mechanism for water oxidation catalysed by these complexes. To understand more about the mechanism we try to study the reaction under different conditions using various different spectroscopic techniques, e.g. EPR spectroscopy and mass spectrometry.
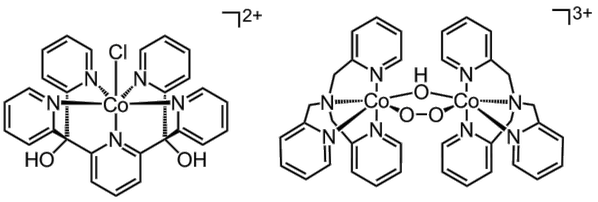
Figure 3. Two examples of cobalt-based catalysts studied in the group.
The mononuclear cobalt complex is currently being modified to be able to anchor to an electrode surface to facilitate the development of photo-anodes.
References
- Shevchenko, D.; Anderlund, M. F.; Thapper, A.; Styring, S. Energy Envir. Sci. 2011, 4, 1284-1287.
- Risch, M.; Shevchenko, D.; Anderlund, M. F.; Styring, S.; Heidkamp, J.; Lange, K. M.; Thapper, A.; Zaharieva, I. Int. J. Hydrogen Energy 2012, 37, 8878-8888.
- Wang, H. Y.; Liu, J.; Zhu, J.; Styring, S.; Ott, S.; Thapper, A. Phys Chem Chem Phys 2014, 16, 3661-3669.
- Wang, H. Y.; Mijangos, E.; Ott, S.; Thapper, A. Angew. Chem. Int. Ed. 2014, 53, 14499-14502.
