Polymer nano-photocatalysts
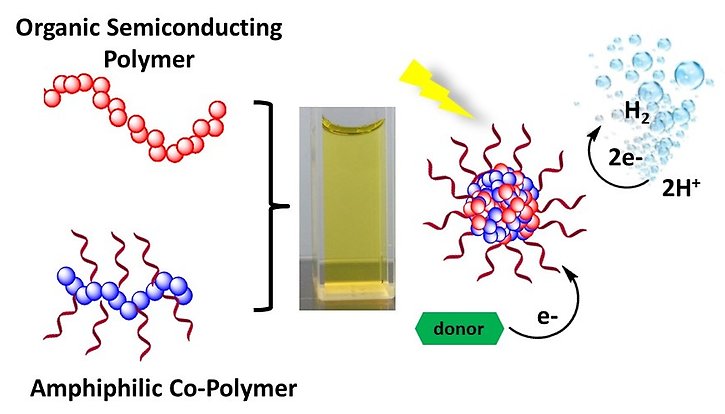
Making organic polymers into Pdots (nano scale dots) can improve the photocatalytic performance.
Organic polymers have potential to obtain the high photocatalytic efficiency, since their structures and corresponding properties can be facilely tuned by constructing them with differing chromophoric building blocks. However, all polymers reported so far for proton reduction are hydrophobic, leading to unsatisfactory performance in pure water. For this reason, the addition of an organic solvent or much liquid electron/proton donor, such as MeOH or triethanolamine (TEOA), is desirable. We experimentally proved that making the organic polymer into nano scale dots (Pdots) can dramatically improve the photocatalytic performance of proton reduction in aqueous solution without addition of any organic solvent.
Relevant publications
- Wang L., Fernández-Terán R., Zhang L., Fernandes D., Tian. L., Chen H., Tian H., Organic Polymer Dots as Photocatalysts for Visible Light-Driven Hydrogen Generation, Angew Chem Int Ed, 2016, 55 (40), 12306-12310.
- Pati P., Damas G., Tian L., Fernandes D., Zhang L., Pehlivan I., Edvinsson T., Araujo C., Tian H. An experimental and theoretical study of an efficient polymer nano-photocatalyst for hydrogen evolution, Energy Environ. Sci., 2017,10, 1372-1376
- Liu A., Tai C., Hola., Tian H. Hollow Polymer Nanoparticles: Nature Mimicking Architecture for Efficient Photocatalytic Hydrogen Evolution, J. Mater. Chem. A, 2019, doi: 10.1039/C8TA12146J
- Liu A., Gedda L., Axelsson M., Pavliuk M., Edwards K., Hammarström L., Tian H. Panchromatic Ternary Polymer Dots Involving Sub-Picosecond Energy and Charge Transfer for Efficient and Stable Photocatalytic Hydrogen Evolution. J. Am. Chem. Soc., 2021, 143, 7, 2875–2885
