Bryndis Birnir
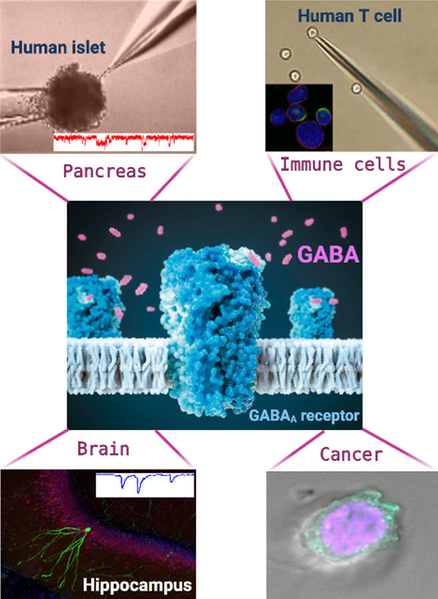
The GABA Lab
The GABA Lab at Uppsala University was established in 2008 by Professor Birnir and focuses on the role of the extracellular signaling molecule GABA (γ-aminobutyric acid) in the brain, in the pancreatic islets and in the blood. In the brain, GABA reduces excitability of neurons and is the main inhibitory neurotransmitter. In the pancreatic islets, GABA modulates insulin and glucagon secretion and promotes maintenance of the cells that make insulin. In the blood, GABA regulates release of both inflammatory and anti-inflammatory molecules from T cells and reduces proliferation of immune cells. GABA appears to be a major homeostatic molecule promoting balance and health. Because of the importance of GABA signaling in physiology, medicines acting on the GABA system are very important in many diseases i.e., psychiatric and neurodegenerative diseases but also epilepsy and diabetes.

Modell av GABA receptor
Metabolic hormones regulate GABA signaling in the brain
It is becoming clear that metabolic hormones like insulin, glucagon-like peptide 1 (GLP-1) and IAPP (islet amyloid polypeptide) facilitate a number of brain functions including motivation and cognition. We have shown that insulin and GLP-1 regulate GABA signaling at synapses and extrasynaptic sites in the brain. And further, that insulin modulates the changing brain during development and in neurodegenerative disease such as Alzheimer’s disease (AD), by regulating the GABA signaling system in neurons. Insulin dysfunction is the principal hallmark of type 2 diabetes mellitus and metabolic dysregulation is a risk factor for cognitive decline, vascular dementia and AD. Our current and previous results on the GABA signaling system in the hippocampus, the center for memory and learning in the brain, support the concept that the effects of metabolic hormones are a critical part of normal brain development, plasticity and homeostasis in health and disease. Whether insulin/GLP-1/IAPP have a role in other brain diseases, e.g. psychiatric disorders or epilepsy, remains to be explored. But considering the wide distribution of e.g. the insulin receptor in the brain, it seems likely that normal signaling by metabolic hormones in the brain is of importance for healthy brain function and may serve to normalize imbalances in a range of brain pathologies. We have a number of on-going projects in this area.
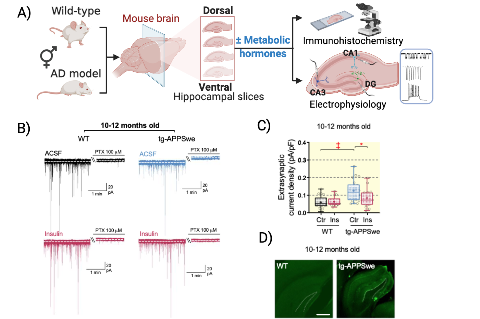
Effects of metabolic hormones on GABAergic inhibition in mouse hippocampus. A) Schematic diagram of the experimental design (Created with Biorender.com). B) Representative GABA-activated current traces from dentate gyrus granule cells from dorsal hippocampal slices from wild-type and tg-APPSwe mice under control conditions (ACSF) and after exposure to 1 nM insulin. Upward shift of the baseline under the application of picrotoxin (PTX, 100 µM) identifies the tonic current amplitude (as the difference between the dashed lines). C) Insulin normalizes increased tonic GABA-activated extrasynaptic current density in dentate gyrus granule cells in 10-12 months old tg-APPSwe mice. The tonic extrasynaptic current density was significantly higher in tg-APPSwe mice when compared with the wild-type littermates. Significance levels are *P < 0.05, ‡P < 0.001. D) Thioflavin S staining showed the presence of neuritic plaques (bright green) in the hippocampus of tg-APPSwe AD but not wild-type mice. Scale bar = 500 µm.
DOI: 10.1111/apha.13623
Selected articles
- Insulin differentially modulates GABA signalling in hippocampal neurons and, in an age-dependent manner, normalizes GABA-activated currents in the tg-APPSwe mouse model of Alzheimer's disease. DOI: 10.1111/apha.13623
- GLP-1 and exendin-4 transiently enhance GABAA receptor-mediated synaptic and tonic currents in rat hippocampal CA3 pyramidal neurons. DOI: 10.2337/db14-0668
- GABA-activated single-channel and tonic currents in rat brain slices. DOI: 10.3791/2858
GABA channels in α-, β-, δ-pancreatic islets cells
We use intact human islets to study how GABA regulates function of the different islet cell-types. GABA is secreted from the β-cells and is in the sub-micromolar range in the interstitial fluids. GABA activates GABA channels in the cells. In type 2 diabetes the GABA channels in b-cells become supersensitive to GABA. We have further showed that a significant proportion of the cells in the human pancreatic islets express transcripts for more than one hormone e.g. insulin, glucagon, somatostatin. Now our focus is on the a cells and the mixed transcripts cells in human islets and how GABA regulates hormone secretion from these cells.
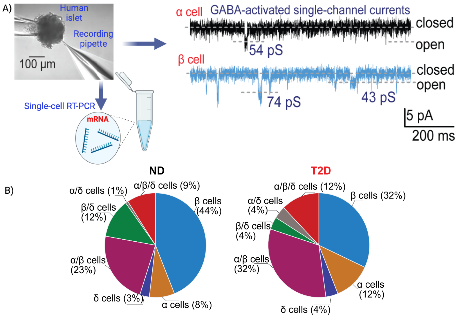
Study of GABA signaling in human pancreatic islet cells. A) Experimental design of the process of single-channel current recording from intact human islet cells followed by single-cell RT-PCR for further identification of the cell-type. The electrophysiological recordings to the right show interstitial GABA-activated currents in α and β cells with representative single-channel conductances (in pS). B) Percentage distribution of single and multiple hormone transcript-expressing cells from non-diabetes (ND) and type 2 diabetes (T2D) donors.
DOI: 10.1016/j.ebiom.2018.03.014
DOI: 10.3390/ijms21020600
Selected articles
- Functional Characterization of Native, High-Affinity GABA A Receptors in Human Pancreatic β Cells. DOI: 10.1016/j.ebiom.2018.03.014
- GABA A Receptor-Mediated Currents and Hormone mRNAs in Cells Expressing More Than One Hormone Transcript in Intact Human Pancreatic Islets. DOI: 10.3390/ijms21020600
- γ-Aminobutyric acid (GABA) signaling in human pancreatic islets is altered in type 2 diabetes. DOI: 10.1007/s00125-012-2548-7
GABA regulates T cells proliferation and cytokine secretion
It was in 2008 that the Birnir Lab demonstrated, for the first time, that high-affinity GABA channels regulate T cells and in 2018, we showed that GABA regulates both proliferation and cytokine secretion from immune cells. We showed that GABA at physiological concentrations is a potent regulator of secretion of both pro- and anti-inflammatory cytokines from peripheral blood mononuclear cells (PBMCs) and T cells, from both healthy and Type 1 diabetes (T1D) donors. Importantly, we identified GABA responder and non-responder cells. Non-responder cells are not inhibited by GABA in the proliferation assay and often already secrete substantial levels of cytokines. Responder cells are those that show decreased proliferation in the presence of GABA. We revealed that the low sub-micromolar GABA concentrations are present in blood from normal donors (ND) and T1D, T2D and psychiatric patients. Nevertheless, the molecular mechanism of GABA modulation of the immune cells e.g. how the GABA regulation of proliferation and cytokine secretion comes about, how the regulation varies among subtypes of T cells and what determines if a cell is a responder or non-responder is not known today and this is what we are focusing on in the Lab.
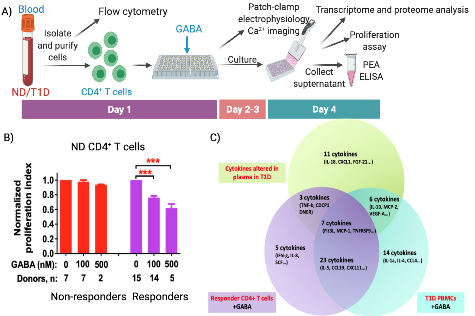
GABA regulates T cells proliferation and cytokine secretion. A) Schematic diagram of the experimental design and time line (Created with Biorender.com). B) Effect of GABA on proliferation of CD4+ T cells from ND donors identifying GABA non-responder and responder populations of CD4+ T cells. Significance level is ***P < 0.001. C) PEA data summary of cytokine profile regulated by GABA and T1D. DOI: 10.1016/j.ebiom.2018.03.019
Selected articles
- GABA Regulates Release of Inflammatory Cytokines from Peripheral Blood Mononuclear Cells and CD4 +T Cells and Is Immunosuppressive in Type 1 Diabetes. DOI: 10.1016/j.ebiom.2018.03.019
- Depression, GABA, and Age Correlate with Plasma Levels of Inflammatory Markers. DOI: 10.3390/ijms20246172
- GABA, a natural immunomodulator of T lymphocytes. DOI: 10.1016/j.jneuroim.2008.08.017
Cancer and relevance of GABA signaling
GABA and GABA receptors (GABAA and GABAB) have been identified in different types of tumor tissues including glioma, pancreatic cancer and breast cancer. The GABA signaling is implicated in cancer cell proliferation and migration as well as immune response within the tumor microenvironment. We have shown that the expression of GABAA r2 subunit is associated with patient survival in diffuse astrocytomas. In addition, we have detected functional GABAA receptors in a human glioblastoma derived cell line that are modulated by GABAergic agents such as diazepam, propofol and etomidate. This suggest GABA signaling is a potential therapeutic target and we are continuing to investigate the mechanism underlying GABA modulation of cancer cells and infiltrated immune cells.
Selected articles
GABA-A channel subunit expression in human glioma correlates with tumor histology and clinical outcome. DOI: 10.1371/journal.pone.0037041
- Etomidate, propofol and diazepam potentiate GABA-evoked GABAA currents in a cell line derived from human glioblastoma. DOI: 10.1016/j.ejphar.2014.12.001
ENABLE
We are part of the in vivo/safety platform -forming the ion channel platform.
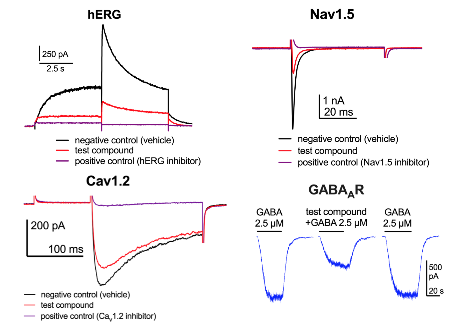
Effects of test compounds on hERG, Nav1.5, Cav1.2 and GABAAR-mediated currents measured by whole-cell patch clamp recordings.
In vitro pharmacological profiling involves the screening of compounds against a broad range of targets such as ion channels, transporters and receptors that are different from the intended therapeutic target (or targets). Ion channels have prominent role in the physiology of all cell-types and drug interactions with ion channels have long been associated with undesired effects. Ion channels of high pharmacological relevance for drug developments are: voltage-gated potassium channel subfamily H member 2 (KCNH2; also known as hERG), voltage-gated sodium (Nav1.5), calcium (Cav1.2), potassium (KCNQ1) channels and ligand-gated receptors like GABAA receptors that are ion channels opened by the neurotransmitter GABA. These different ion channels are very important for i.e. cardiac, neuronal and endocrine functions. Within the consortium we have tested if newly developed compounds interact with these ion channels.
http://nd4bb-enable.eu/consortium
ENABLE: an engine for European antibacterial drug discovery and development https://www.nature.com/articles/d41573-021-00074-y
Methods
More info on established methods, instruments and techniques available in the group.
Publications
Part of Journal of Medicinal Chemistry, p. 1380-1425, 2023
- DOI for Discovery and Hit-to-Lead Optimization of Benzothiazole Scaffold- Based DNA Gyrase Inhibitors with Potent Activity against Acinetobacter baumannii and Pseudomonas aeruginosa
- Download full text (pdf) of Discovery and Hit-to-Lead Optimization of Benzothiazole Scaffold- Based DNA Gyrase Inhibitors with Potent Activity against Acinetobacter baumannii and Pseudomonas aeruginosa
Part of Biomedicines, 2022
- DOI for Endogenous Levels of Gamma Amino-Butyric Acid Are Correlated to Glutamic-Acid Decarboxylase Antibody Levels in Type 1 Diabetes
- Download full text (pdf) of Endogenous Levels of Gamma Amino-Butyric Acid Are Correlated to Glutamic-Acid Decarboxylase Antibody Levels in Type 1 Diabetes
Part of Frontiers in Molecular Neuroscience, 2022
- DOI for ZEB2 haploinsufficient Mowat-Wilson syndrome induced pluripotent stem cells show disrupted GABAergic transcriptional regulation and function
- Download full text (pdf) of ZEB2 haploinsufficient Mowat-Wilson syndrome induced pluripotent stem cells show disrupted GABAergic transcriptional regulation and function
Part of Acta Physiologica, 2021
- DOI for Insulin differentially modulates GABA signalling in hippocampal neurons and, in an age-dependent manner, normalizes GABA-activated currents in the tg-APPSwe mouse model of Alzheimer's disease
- Download full text (pdf) of Insulin differentially modulates GABA signalling in hippocampal neurons and, in an age-dependent manner, normalizes GABA-activated currents in the tg-APPSwe mouse model of Alzheimer's disease
Part of American Journal of Human Genetics, p. 739-748, 2021
- DOI for Monoallelic and bi-allelic variants in NCDN cause neurodevelopmental delay, intellectual disability, and epilepsy
- Download full text (pdf) of Monoallelic and bi-allelic variants in NCDN cause neurodevelopmental delay, intellectual disability, and epilepsy
Part of International Journal of Molecular Sciences, 2020
- DOI for GABA(A) Receptor-Mediated Currents and Hormone mRNAs in Cells Expressing More Than One Hormone Transcript in Intact Human Pancreatic Islets
- Download full text (pdf) of GABA(A) Receptor-Mediated Currents and Hormone mRNAs in Cells Expressing More Than One Hormone Transcript in Intact Human Pancreatic Islets
Part of Hippocampus, p. 1146-1157, 2020
- DOI for Tonic GABA-activated synaptic and extrasynaptic currents in dentate gyrus granule cells and CA3 pyramidal neurons along the mouse hippocampal dorsoventral axis
- Download full text (pdf) of Tonic GABA-activated synaptic and extrasynaptic currents in dentate gyrus granule cells and CA3 pyramidal neurons along the mouse hippocampal dorsoventral axis
Part of Acta Physiologica, 2019
Part of Neurobiology of Disease, 2019
- DOI for Transcriptomes of Dravet syndrome iPSC derived GABAergic cells reveal dysregulated pathways for chromatin remodeling and neurodevelopment
- Download full text (pdf) of Transcriptomes of Dravet syndrome iPSC derived GABAergic cells reveal dysregulated pathways for chromatin remodeling and neurodevelopment
Part of Journal of Neuroimmunology, 2019
- DOI for Interferon-gamma potentiates GABA(A) receptor-mediated inhibitory currents in rat hippocampal CA1 pyramidal neurons
- Download full text (pdf) of Interferon-gamma potentiates GABA(A) receptor-mediated inhibitory currents in rat hippocampal CA1 pyramidal neurons
Depression, GABA, and Age Correlate with Plasma Levels of Inflammatory Markers
Part of International Journal of Molecular Sciences, 2019
- DOI for Depression, GABA, and Age Correlate with Plasma Levels of Inflammatory Markers
- Download full text (pdf) of Depression, GABA, and Age Correlate with Plasma Levels of Inflammatory Markers
Ataxia in Patients With Bi-Allelic NFASC Mutations and Absence of Full-Length NF186
Part of Frontiers in Genetics, 2019
- DOI for Ataxia in Patients With Bi-Allelic NFASC Mutations and Absence of Full-Length NF186
- Download full text (pdf) of Ataxia in Patients With Bi-Allelic NFASC Mutations and Absence of Full-Length NF186
Part of Acta Physiologica, 2018
Part of EBioMedicine, p. 283-294, 2018
- DOI for GABA Regulates Release of Inflammatory Cytokines From Peripheral Blood Mononuclear Cells and CD4+ T Cells and Is Immunosuppressive in Type 1 Diabetes
- Download full text (pdf) of GABA Regulates Release of Inflammatory Cytokines From Peripheral Blood Mononuclear Cells and CD4+ T Cells and Is Immunosuppressive in Type 1 Diabetes
Functional Characterization of Native, High-Affinity GABAA Receptors in Human Pancreatic β Cells
Part of EBioMedicine, 2018
- DOI for Functional Characterization of Native, High-Affinity GABAA Receptors in Human Pancreatic β Cells
- Download full text (pdf) of Functional Characterization of Native, High-Affinity GABAA Receptors in Human Pancreatic β Cells
Part of PLOS ONE, 2018
- DOI for Expression of calcium release-activated and voltage-gated calcium channels genes in peripheral blood mononuclear cells is altered in pregnancy and in type 1 diabetes
- Download full text (pdf) of Expression of calcium release-activated and voltage-gated calcium channels genes in peripheral blood mononuclear cells is altered in pregnancy and in type 1 diabetes
Insulin enhances GABAA receptor-mediated inhibitory currents in rat central amygdala neurons
Part of Neuroscience Letters, p. 76-81, 2018
- DOI for Insulin enhances GABAA receptor-mediated inhibitory currents in rat central amygdala neurons
- Download full text (pdf) of Insulin enhances GABAA receptor-mediated inhibitory currents in rat central amygdala neurons
Part of Journal of Neuroimmunology, p. 51-58, 2017
Part of BMC Pharmacology & Toxicology, 2017
- DOI for Liraglutide modulates GABAergic signaling in rat hippocampal CA3 pyramidal neurons predominantly by presynaptic mechanism
- Download full text (pdf) of Liraglutide modulates GABAergic signaling in rat hippocampal CA3 pyramidal neurons predominantly by presynaptic mechanism
Part of Neuroscience, p. 300-321, 2017
- DOI for Characterization of the gamma-aminobutyric acid signaling system in the zebrafish (danio rerio hamilton) central nervous system by reverse transcription-quantitative polymerase chain reaction
- Download full text (pdf) of Characterization of the gamma-aminobutyric acid signaling system in the zebrafish (danio rerio hamilton) central nervous system by reverse transcription-quantitative polymerase chain reaction
Part of Journal of neural transmission, p. 137-157, 2016
Part of Diabetes, p. 79-89, 2015
- DOI for GLP-1 and Exendin-4 Transiently Enhance GABA(A) Receptor-Mediated Synaptic and Tonic Currents in Rat Hippocampal CA3 Pyramidal Neurons
- Download full text (pdf) of GLP-1 and Exendin-4 Transiently Enhance GABA(A) Receptor-Mediated Synaptic and Tonic Currents in Rat Hippocampal CA3 Pyramidal Neurons
Part of Acta Physiologica, p. 91-91, 2015
Part of Acta Physiologica, p. 89-90, 2015
GABAergic signalling in the immune system
Part of Acta Physiologica, p. 819-827, 2015
Part of European Journal of Pharmacology, p. 101-107, 2015
Part of Acta Physiologica, p. 575-585, 2015
Part of PLOS ONE, 2015
- DOI for The GLP-1 Receptor Agonist Exendin-4 and Diazepam Differentially Regulate GABAA Receptor-Mediated Tonic Currents in Rat Hippocampal CA3 Pyramidal Neurons
- Download full text (pdf) of The GLP-1 Receptor Agonist Exendin-4 and Diazepam Differentially Regulate GABAA Receptor-Mediated Tonic Currents in Rat Hippocampal CA3 Pyramidal Neurons
Insulin modulates GABAA receptor-mediated neuronal inhibition in rat hippocampus and amygdala
Part of Acta Physiologica, p. 90-90, 2015
Absence of Shb impairs insulin secretion by elevated FAK activity in pancreatic islets
Part of Journal of Endocrinology, p. 267-275, 2014
- DOI for Absence of Shb impairs insulin secretion by elevated FAK activity in pancreatic islets
- Download full text (pdf) of Absence of Shb impairs insulin secretion by elevated FAK activity in pancreatic islets
Part of Acta Physiologica, p. 100-100, 2014
GABA-A receptor subunit expression in human peripheral blood mononuclear cells
Part of Acta Physiologica, p. 86-86, 2014
Part of Acta Physiologica, p. 83-83, 2014
Insulin modulates GABA(A) receptor-mediated inhibition in rat amygdala neurons
Part of Acta Physiologica, p. 83-83, 2014
Part of Frontiers in Cellular Neuroscience, p. 288, 2014
Part of Frontiers in Cellular Neuroscience, p. 11, 2014
Part of Frontiers in Cellular Neuroscience, 2014
Monoacylglycerols Activate TRPV1-A Link between Phospholipase C and TRPV1
Part of PLOS ONE, 2013
- DOI for Monoacylglycerols Activate TRPV1-A Link between Phospholipase C and TRPV1
- Download full text (pdf) of Monoacylglycerols Activate TRPV1-A Link between Phospholipase C and TRPV1
Part of PLOS ONE, 2013
- DOI for In Intact Islets Interstitial GABA Activates GABA(A) Receptors That Generate Tonic Currents in alpha-Cells
- Download full text (pdf) of In Intact Islets Interstitial GABA Activates GABA(A) Receptors That Generate Tonic Currents in alpha-Cells
GABA is an effective immunomodulatory molecule
Part of Amino Acids, p. 87-94, 2013
gamma-Aminobutyric acid (GABA) signalling in human pancreatic islets is altered in type 2 diabetes
Part of Diabetologia, p. 1985-1994, 2012
Part of PLoS pathogens, 2012
- DOI for GABAergic Signaling Is Linked to a Hypermigratory Phenotype in Dendritic Cells Infected by Toxoplasma gondii
- Download full text (pdf) of GABAergic Signaling Is Linked to a Hypermigratory Phenotype in Dendritic Cells Infected by Toxoplasma gondii
Part of PLOS ONE, 2012
- DOI for GABA-A Channel Subunit Expression in Human Glioma Correlates with Tumor Histology and Clinical Outcome
- Download full text (pdf) of GABA-A Channel Subunit Expression in Human Glioma Correlates with Tumor Histology and Clinical Outcome
Part of PLOS ONE, 2012
- DOI for GABA maintains the proliferation of progenitors in the developing chick ciliary marginal zone and non-pigmented ciliary epithelium:
- Download full text (pdf) of GABA maintains the proliferation of progenitors in the developing chick ciliary marginal zone and non-pigmented ciliary epithelium:
Different subtypes of GABA-A receptors are expressed in human, mouse and rat T lymphocytes
Part of PLOS ONE, 2012
- DOI for Different subtypes of GABA-A receptors are expressed in human, mouse and rat T lymphocytes
- Download full text (pdf) of Different subtypes of GABA-A receptors are expressed in human, mouse and rat T lymphocytes
Part of European Journal of Neurology, p. 67-67, 2012
GABA is an efficient immunomodulator molecule
Part of Nerve-Driven Immunity, p. 163-173, Springer, 2012
Part of Frontiers in Cellular Neuroscience, 2012
- DOI for Selective Changes of GABA(A) Channel Subunit mRNAs in the Hippocampus and Orbitofrontal Cortex but not in Prefrontal Cortex of Human Alcoholics
- Download full text (pdf) of Selective Changes of GABA(A) Channel Subunit mRNAs in the Hippocampus and Orbitofrontal Cortex but not in Prefrontal Cortex of Human Alcoholics
Part of Molecular Immunology, p. 399-407, 2011
Insulin reduces neuronal excitability by turning on GABA(A) channels that generate tonic current
Part of PLOS ONE, 2011

