Joey Lau Börjesson
Bioengineering strategies to create local protection of ß-cells after transplantation
My research has over the years been focused on β-cell physiology and β-cell replacement as therapy for type 1 diabetes. A main obstacle in β-cell replacement is immunological components hampering graft survival, where innate immune, autoimmune and allogeneic components all contribute. The research in our group is focused on creating local protection from cellular death combined with the induction of local tolerance. Thereby, the opportunity to exclude immunosuppressive drugs from β-cell replacement therapies may be achieved. This would then open the possibility for such treatment for the majority of T1D patients. Since there is a limited number of β-cells available from conventional organ donors for clinical transplantation, protocols are focused on using a renewable source of insulin-producing cells derived from human pluripotent stem cells. In our lab, we generate insulin-producing islet-like cell clusters from induced pluripotent stem cells and embryonic stem cells. The former also provides the opportunity to circumvent allogeneic reactions focusing on innate immune and possible autoimmune components. The strategies included are to diminish innate immunity reactions after transplantation by using biomaterials and/or to coat islets with protective cells such as decidual or mesenchymal stem cells before transplantation. A challenge in this effort is to identify strategies that are clinically translatable, i.e. find a modification that could be accepted as an advanced therapeutic medicinal product (ATMP).
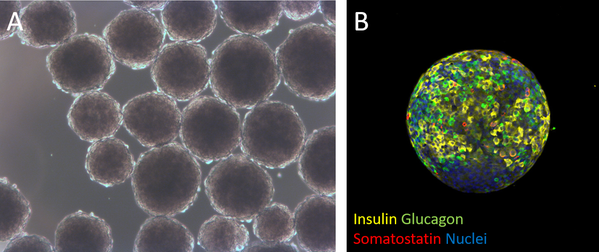
Generation of insulin-producing islet-like cell clusters derived from human pluripotent stem cells Figure A. Human pluripotent stem cell-derived islet-like cell clusters in culture. Figure B. Immunostained islet-like cell cluster derived from human pluripotent stem cells.
Publications
Part of Frontiers in Membrane Science and Technology, 2024
- DOI for Differentiation of human pluripotent stem cells into insulin-producing islet-like clusters using nanofiltered cell culture medium
- Download full text (pdf) of Differentiation of human pluripotent stem cells into insulin-producing islet-like clusters using nanofiltered cell culture medium
CLEC11A improves insulin secretion and promotes cell proliferation in human beta-cells
Part of Journal of Molecular Endocrinology, 2023
- DOI for CLEC11A improves insulin secretion and promotes cell proliferation in human beta-cells
- Download full text (pdf) of CLEC11A improves insulin secretion and promotes cell proliferation in human beta-cells
Part of Free radical research, p. 460-469, 2023
- DOI for The selective NOX4 inhibitor GLX7013159 decreases blood glucose concentrations and human beta-cell apoptotic rates in diabetic NMRI nu/nu mice transplanted with human islets
- Download full text (pdf) of The selective NOX4 inhibitor GLX7013159 decreases blood glucose concentrations and human beta-cell apoptotic rates in diabetic NMRI nu/nu mice transplanted with human islets
Part of EJNMMI Research, p. 107, 2023
- DOI for Preclinical evaluation of Affibody molecule for PET imaging of human pancreatic islets derived from stem cells.
- Download full text (pdf) of Preclinical evaluation of Affibody molecule for PET imaging of human pancreatic islets derived from stem cells.
Part of Nature Biotechnology, p. 1042-1055, 2022
- DOI for Functional, metabolic and transcriptional maturation of human pancreatic islets derived from stem cells.
- Download full text (pdf) of Functional, metabolic and transcriptional maturation of human pancreatic islets derived from stem cells.
Part of Stem Cell Research, 2021
- DOI for Generation of human induced pluripotent stem cell (iPSC) lines (UUMCBi001-A, UUMCBi002-A) from two healthy donors
- Download full text (pdf) of Generation of human induced pluripotent stem cell (iPSC) lines (UUMCBi001-A, UUMCBi002-A) from two healthy donors
Part of International Journal of Molecular Sciences, 2021
- DOI for Interleukin-35 Prevents Development of Autoimmune Diabetes Possibly by Maintaining the Phenotype of Regulatory B Cells
- Download full text (pdf) of Interleukin-35 Prevents Development of Autoimmune Diabetes Possibly by Maintaining the Phenotype of Regulatory B Cells
Part of Nature Communications, 2021
- DOI for Agonistic CD40 therapy induces tertiary lymphoid structures but impairs responses to checkpoint blockade in glioma
- Download full text (pdf) of Agonistic CD40 therapy induces tertiary lymphoid structures but impairs responses to checkpoint blockade in glioma
Function and Gene Expression of Islets Experimentally Transplanted to Muscle and Omentum
Part of Cell Transplantation, p. 1-10, 2020
- DOI for Function and Gene Expression of Islets Experimentally Transplanted to Muscle and Omentum
- Download full text (pdf) of Function and Gene Expression of Islets Experimentally Transplanted to Muscle and Omentum
Part of Experimental Cell Research, 2019
Part of Cell Transplantation, p. 1455-1460, 2019
- DOI for Fewer Islets Survive from a First Transplant than a Second Transplant: Evaluation of Repeated Intraportal Islet Transplantation in Mice
- Download full text (pdf) of Fewer Islets Survive from a First Transplant than a Second Transplant: Evaluation of Repeated Intraportal Islet Transplantation in Mice
Part of Upsala Journal of Medical Sciences, p. 228-237, 2019
- DOI for Characterization of neural crest-derived stem cells isolated from human bone marrow for improvement of transplanted islet function.
- Download full text (pdf) of Characterization of neural crest-derived stem cells isolated from human bone marrow for improvement of transplanted islet function.
Part of Journal of the Endocrine Society, p. 1608-1616, 2019
- DOI for Decreased beta-Cell Proliferation and Vascular Density in a Subpopulation of Low-Oxygenated Male Rat Islets
- Download full text (pdf) of Decreased beta-Cell Proliferation and Vascular Density in a Subpopulation of Low-Oxygenated Male Rat Islets
Part of Diabetes Therapy, p. 1511-1532, 2018
- DOI for A Randomized Controlled Trial of Dapagliflozin Plus Once-Weekly Exenatide Versus Placebo in Individuals with Obesity and Without Diabetes: Metabolic Effects and Markers Associated with Bodyweight Loss
- Download full text (pdf) of A Randomized Controlled Trial of Dapagliflozin Plus Once-Weekly Exenatide Versus Placebo in Individuals with Obesity and Without Diabetes: Metabolic Effects and Markers Associated with Bodyweight Loss
Part of Hormone and Metabolic Research, p. 627-639, 2018
Part of Scientific Reports, 2018
- DOI for Increased Expression of GLP-1R in Proliferating Islets of Men1 Mice is Detectable by [Ga-68]Ga-DO3A-VS-Cys(40)- Exendin-4/PET
- Download full text (pdf) of Increased Expression of GLP-1R in Proliferating Islets of Men1 Mice is Detectable by [Ga-68]Ga-DO3A-VS-Cys(40)- Exendin-4/PET
Towards the clinical translation of stem cell therapy for type 1 diabetes
Part of European Journal of Endocrinology, 2017
Part of Diabetologia, 2017
Part of Pain, p. 945-961, 2017
- DOI for Spinal Cord Interneurons Expressing the Gastrin-Releasing Peptide Receptor Convey Itch Through VGLUT2-Mediated Signaling
- Download full text (pdf) of Spinal Cord Interneurons Expressing the Gastrin-Releasing Peptide Receptor Convey Itch Through VGLUT2-Mediated Signaling
Part of Endocrine, p. 839-852, 2017
- DOI for Role of cannabinoid receptor 1 in human adipose tissue for lipolysis regulation and insulin resistance
- Download full text (pdf) of Role of cannabinoid receptor 1 in human adipose tissue for lipolysis regulation and insulin resistance
Part of Diabetologia, 2016
Part of Diabetologia, 2016
Part of Diabetologia, 2016
Part of Journal of Diabetes Research, 2016
- DOI for Small Mouse Islets Are Deficient in Glucagon-Producing Alpha Cells but Rich in Somatostatin-Secreting Delta Cells
- Download full text (pdf) of Small Mouse Islets Are Deficient in Glucagon-Producing Alpha Cells but Rich in Somatostatin-Secreting Delta Cells
Gastric bypass reduces symptoms and hormonal responses to hypoglycemia
Part of Diabetes, p. 2667-2675, 2016
Part of EMBO Molecular Medicine, p. 729-744, 2016
- DOI for Endoplasmic reticulum stress enhances fibrosis through IRE1α-mediated degradation of miR-150 and XBP-1 splicing
- Download full text (pdf) of Endoplasmic reticulum stress enhances fibrosis through IRE1α-mediated degradation of miR-150 and XBP-1 splicing
Pancreatic islet blood flow and its measurement
Part of Upsala Journal of Medical Sciences, p. 81-95, 2016
- DOI for Pancreatic islet blood flow and its measurement
- Download full text (pdf) of Pancreatic islet blood flow and its measurement
Part of Molecular and Cellular Endocrinology, p. 124-132, 2016
Scholarly publishing threatened?
Part of Upsala Journal of Medical Sciences, p. 205-206, 2016
Part of Diabetologia, 2015
Part of Cell Transplantation, p. 2263-2272, 2015
- DOI for Surface Coating of Pancreatic Islets With Neural Crest Stem Cells Improves Engraftment and Function After Intraportal Transplantation
- Download full text (pdf) of Surface Coating of Pancreatic Islets With Neural Crest Stem Cells Improves Engraftment and Function After Intraportal Transplantation
Increased levels of irisin in people with long-standing Type1 diabetes
Part of Diabetic Medicine, p. 1172-1176, 2015
Operating in an era of impact factor mania
Part of Upsala Journal of Medical Sciences, p. 124-131, 2015
Part of Transplantation, p. 2077-2082, 2015
Part of Diabetologia, p. 132-139, 2015
Part of International Journal of Neuroscience, p. 547-554, 2015
Part of Genome Research, p. 1521-1535, 2015
- DOI for Signatures of post-zygotic structural genetic aberrations in the cells of histologically normal breast tissue that can predispose to sporadic breast cancer
- Download full text (pdf) of Signatures of post-zygotic structural genetic aberrations in the cells of histologically normal breast tissue that can predispose to sporadic breast cancer
Part of Journal of Gastroenterology, Pancreatology & Liver Disorders, p. 1-9, 2014
Part of Journal of endocrinology and diabetes mellitus, p. 65-69, 2014
- DOI for Improving Pancreatic Islet Engraftment after Islet Transplantation through Administration of Gamma-Secretase Inhibitor DAPT
- Download full text (pdf) of Improving Pancreatic Islet Engraftment after Islet Transplantation through Administration of Gamma-Secretase Inhibitor DAPT
Increased circulating levels of betatrophin in individuals with long-standing type 1 diabetes
Part of Diabetologia, p. 50-53, 2014
Part of Transplantation, 2013
Part of Transplantation, 2013
Part of Transplantation, 2013
Part of Transplantation, 2013
Part of Transplantation, 2013
Part of Pancreas, p. 1263-1271, 2012
Part of Diabetes, p. 665-673, 2012
Part of Diabetologia, p. 1390-1399, 2012
Striated Muscle as Implantation Site for Transplanted Pancreatic Islets
Part of Journal of Transplantation, p. 352043, 2011
Part of Cell Transplantation, p. 783-788, 2011

