Fredrik Palm
Renal physiology and kidney disease
Kidney disease is a major cause for premature mortality. Recently, the focus has shifted from a glomerulocentric to a predominantly tubulocentric view of mechanisms involved in the development of kidney disease caused by diabetes, hypertension or acute kidney injury (Vallon and Thomson 2012). Our research program focuses on better understanding of basic renal physiology and pathological mechanisms leading to kidney disease. Recently, we have directed substantial efforts towards delineating the involvement of deranged kidney oxygen homeostasis for the onset and progression of acute and chronic kidney disease.
Kidney tissue oxygen tension is low already under normal conditions (Aukland and Krog 1960, Leichtweiss et al 1969) and attempts to increase oxygen delivery via increased renal blood flow normally also result in augmented tubular load of electrolytes due to elevated glomerular filtration rate, which in itself increases the metabolic demand. Thus, any increase in kidney metabolism is likely to result in decreased kidney tissue oxygen tension, i.e. hypoxia. Indeed, increased kidney metabolism is associated with diabetic nephropathy (Korner et al 1994) and diabetes is associated with a decreased kidney tissue oxygen tension in both animals and patients (Ries et al 2003, dos Santos et al 2007, Rosenberger et al 2008, Edlund et al 2009, Haidara et al. 2009, Inoue et al. 2011). Fine et al. proposed that an initial glomerular injury decreases blood flow through peritubular capillaries and results in decreased oxygenation of the kidney, promoting tubulointerstitial fibrosis and progression to kidney damage (Fine, Orphanides et al 1998). Importantly, chronic tubulointerstitial hypoxia has been proposed as a common pathway to end stage renal disease irrespective of initial insult (Nangaku 2004, Nangaku 2006, Singh et al 2008, Mimura and Nangaku 2010, Palm and Nordquist 2011).
In 2003, we presented our first report demonstrating intrarenal tissue hypoxia in diabetes (Palm et al. 2003), which after that has been verified by several international laboratories (Ries et al 2003, dos Santos et al 2007, Rosenberger et al 2008, Yin et al 2012). Since then we have continued to investigate the role of kidney tissue hypoxia for the development of diabetic nephropathy, but also during other conditions associated with increased risk of kidney disease, such as hypertension, ischemia-reperfusion injury and surgical 5/6 nephrectomy.
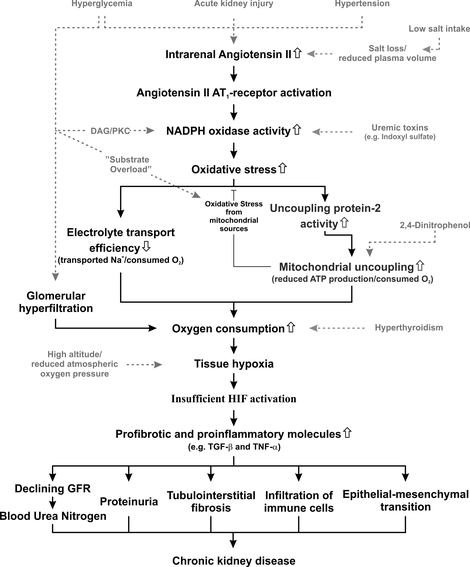
Unifying hypothesis for the lab’s activities. Most pathways have already been published. A few of the pathways listed in this overall scheme are currently only supported by unpublished data, but are to be published shortly.
NADPH oxidases and diabetic kidney disease
Diabetes results in a decreased coupling between oxygen consumption and ATP production meaning that more oxygen is required to produce the same amount of ATP. This mismatch between oxygen consumption and ATP production is initially driven by regulated activation of the mitochondria protein uncoupling protein 2 (UCP-2). This is a proton carrier that translocate protons over the inner membrane in the mitochondria bypassing the ATP-synthase and therefore causes reduced efficiency. UCP-2 is directly activated by superoxide radicals. Indeed treatment with antioxidants has been shown to prevent activation of UCP-2 and thereby prevent proton leakage. The source of superoxide radicals activating UCP-2 in diabetes can involve the mitochondria itself or be the results of activation of NADPH oxidases. NADPH oxidases are enzymes responsible for a regulated production of superoxide radicals and hydrogen peroxide required for normal cell signaling. In the kidney the isoforms NADPH oxidase 1, 2 and 4 are present (also isoform 5 in humans). In diabetes, these enzymes are activated which causes increased production of superoxide radicals. However, it is presently not know if there is a direct link between NADPH oxidase activity and UCP-2-mediated proton leakage. The current project investigate a putative signaling between NADPH oxidases and mitochondria efficiency, utilizing primary culture of renal epithelial proximal tubular cells from wild-type and UCP-2 knockout mice. Novel NADPH oxidase inhibitors and siRNA mediated knockdown is used for modulating NADPH oxidase activity in isolated cells followed by detailed evaluation of oxygen consumption by high-resolution respirometry.
Mitochondria function in kidney disease
Oxidative phosphorylation is essential in order to provide sufficient cellular energy to sustain tubular function and, thus, whole-body homeostasis. We have reported altered mitochondria function in the diabetic kidney and we are now investigating its relationship to kidney disease. Mitochondrial function and hydrogen peroxide production can be measured in isolated mitochondria and permeabilized cells using high-resolution respirometry. This is performed in the Oroboros Oxygraph 2k by adding respiratory complex-specific substrates and inhibitors in order to investigate the intrinsic relative and absolute activities of the different complexes and also determine the mitochondrial oxygen affinity, maximal uncoupled respiration and efficiency (P/O ratio). P/O ratio is measured by steady state infusion of ADP at submaximal respiration (Fig. 1.) that better reflects the in vivo situation compared to the commonly used method by adding a bolus dose of ADP. This approach also allows us to investigate unspecific and protein-mediated proton leak across the inner membrane. The fluorescence module of the Oxygraph 2k allows for simultaneous detection of mitochondrial hydrogen peroxide production during the different respiratory states.
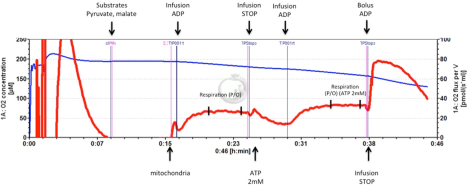
Measuring mitochondrial P/O ratio (±2 mmol/L ATP) by using steady state infusion of ADP at submaximal respiration rate. Blue line: oxygen concentration in the chamber (left scale). Red line: oxygen consumption (right scale). Infusion of ADP in the presence of pyruvate and malate. A bolus dose of ADP is added at the end of the experiment in order to confirm that respiration during the ADP infusion was approximately half-maximum.
Intrarenal Hyaluronan in the Regulation of Fluid Balance. Pathophysiological Relevance to Renal Damage during Diabetes and Ischemia-Reperfusion.
The kidney is a main determinant of fluid/electrolyte balance and of mean arterial blood pressure. Hypertension is often caused by a renal inability to regulate fluid balance. The present research proposal focus on a matrix component (hyaluronan, HA) with extreme water attracting properties in the regulation of fluid balance. The proinflammatory property of HA is also evaluated in pathophysiological models. In contrast to the renal cortex which is almost void of HA, the interstitium of the renal medulla contains high amounts of HA during normal physiological conditions which changes depending on the body hydration status and, more severely, during pathological conditions.
We have found that HA has an important dynamic role in normal renal water-handling (hydration/dehydration) and that the intrarenal distribution of HA is severely altered during diabetes and after ischemia-reperfusion injury which correlates to renal dysfunction and inflammation. The normal intrarenal distribution of HA is also severely altered if angiotensin II tonus is diminished neonatally (during nephrogenesis) in the rat which correlates to renal dysfunction and inflammation.
We aim to:
- determine the physiological relevance of the glycosaminoglycan hyaluronan (HA) in the regulation of renal fluid/electrolyte balance;
- determine the pathophysiological relevance of HA in the renal dysfunction during diabetes (diabetic nephropathy) and after ischemia-reperfusion injury;
- determine if hyaluronidase-treatment and siRNA improves renal function during diabetic nephropathy and following renal ischemia-reperfusion;
- elucidate the time frame and mechanisms in the development of the intrarenal heterogenous distribution of HA which occur neonatally in the rat and its angiotensin II dependency.
Both in vivo and in vitro experiments are performed. Diabetes, ischemia, hydration, dehydration, hormones, pharmacological and biomolecular intervention activate/deactivate the systems. Human renal tissue from resections are also analysed. Rats and genetically modified mice are used during in vivo conditions whereafter the renal tissue is analysed using molecular biology to follow HA (amount, size), HA synthases, hyaluronidases and CD44 expression. Renomedullary interstitial cells in culture are used in parallel to follow similar parameters during interventions. In cooperation with the section of diagnostic radiology (assoc prof Per Liss) the mechanisms underlying diabetic nephropathy is to be validated and the increased sensitivity of the diabetic kidney to radiological contrast agents is elucidated. Cardiovascular disease is a dominant cause for invalidity and mortality. The results of the present projects may give rise to basic understanding of, and new treatment modalities in, fluid balance disorders and cardiovascular diseases.
Methods
Total renal blood flow, non-invasive high resolution magnetic resonance imaging, high-resolution respirometry, wire myography, electron paramagnetic detection of free radicals.
Publications
Part of American Journal of Physiology. Regulatory Integrative and Comparative Physiology, 2023
Dose-dependent regulation of kidney mitochondrial function by angiotensin II
Part of Upsala Journal of Medical Sciences, 2023
- DOI for Dose-dependent regulation of kidney mitochondrial function by angiotensin II
- Download full text (pdf) of Dose-dependent regulation of kidney mitochondrial function by angiotensin II
Renal mitochondrial dysfunction in ovine experimental sepsis-associated acute kidney injury
Part of American Journal of Physiology - Renal Physiology, p. 571-580, 2023
- DOI for Renal mitochondrial dysfunction in ovine experimental sepsis-associated acute kidney injury
- Download full text (pdf) of Renal mitochondrial dysfunction in ovine experimental sepsis-associated acute kidney injury
Part of Critical Care, 2022
- DOI for Decreased renal perfusion during acute kidney injury in critical COVID-19 assessed by magnetic resonance imaging: a prospective case control study
- Download full text (pdf) of Decreased renal perfusion during acute kidney injury in critical COVID-19 assessed by magnetic resonance imaging: a prospective case control study
Part of PLOS ONE, 2022
- DOI for Thyroid hormone increases oxygen metabolism causing intrarenal tissue hypoxia; a pathway to kidney disease
- Download full text (pdf) of Thyroid hormone increases oxygen metabolism causing intrarenal tissue hypoxia; a pathway to kidney disease
Part of eLIFE, 2022
- DOI for Repression of hypoxia-inducible factor-1 contributes to increased mitochondrial reactive oxygen species production in diabetes
- Download full text (pdf) of Repression of hypoxia-inducible factor-1 contributes to increased mitochondrial reactive oxygen species production in diabetes
Mitochondrial Respiration-Dependent ANT2-UCP2 Interaction
Part of Frontiers in Physiology, 2022
- DOI for Mitochondrial Respiration-Dependent ANT2-UCP2 Interaction
- Download full text (pdf) of Mitochondrial Respiration-Dependent ANT2-UCP2 Interaction
Part of Acta Physiologica, 2021
- DOI for Pharmacological HIF-PHD inhibition reduces renovascular resistance and increases glomerular filtration by stimulating nitric oxide generation
- Download full text (pdf) of Pharmacological HIF-PHD inhibition reduces renovascular resistance and increases glomerular filtration by stimulating nitric oxide generation
Part of Clinical Science, p. 2243-2263, 2021
A model of mitochondrial O-2 consumption and ATP generation in rat proximal tubule cells
Part of American Journal of Physiology - Renal Physiology, 2020
Part of Journal of Physiology, p. 5573-5587, 2020
- DOI for Determinants of renal oxygen metabolism during low Na+ diet: effect of angiotensin II AT1 and aldosterone receptor blockade
- Download full text (pdf) of Determinants of renal oxygen metabolism during low Na+ diet: effect of angiotensin II AT1 and aldosterone receptor blockade
Part of American Journal of Physiology - Renal Physiology, 2020
Intrarenal oxygenation determines kidney function during the recovery from an ischemic insult
Part of American Journal of Physiology - Renal Physiology, 2020
Erik Persson (1941-2020): a Remembrance
Part of Acta Physiologica, 2020
Part of Diabetes/Metabolism Research Reviews, 2019
Part of Acta Physiologica, 2019
Metabolite aberrations in early diabetes detected in rat kidney using mass spectrometry imaging
Part of Analytical and Bioanalytical Chemistry, p. 2809-2816, 2019
- DOI for Metabolite aberrations in early diabetes detected in rat kidney using mass spectrometry imaging
- Download full text (pdf) of Metabolite aberrations in early diabetes detected in rat kidney using mass spectrometry imaging
Part of Tomography, p. 239-247, 2019
- DOI for High Intrarenal Lactate Production Inhibits the Renal Pseudohypoxic Response to Acutely Induced Hypoxia in Diabetes
- Download full text (pdf) of High Intrarenal Lactate Production Inhibits the Renal Pseudohypoxic Response to Acutely Induced Hypoxia in Diabetes
Part of American Journal of Physiology - Renal Physiology, 2019
Role of carbonic anhydrase in acute recovery following renal ischemia reperfusion injury
Part of PLOS ONE, 2019
- DOI for Role of carbonic anhydrase in acute recovery following renal ischemia reperfusion injury
- Download full text (pdf) of Role of carbonic anhydrase in acute recovery following renal ischemia reperfusion injury
Acute renal metabolic effect of metformin assessed with hyperpolarised MRI in rats
Part of Diabetologia, p. 445-454, 2018
Part of American Journal of Physiology - Renal Physiology, 2018
Part of Acta Physiologica, 2018
The effect of inactin on kidney mitochondrial function and production of reactive oxygen species
Part of PLOS ONE, 2018
- DOI for The effect of inactin on kidney mitochondrial function and production of reactive oxygen species
- Download full text (pdf) of The effect of inactin on kidney mitochondrial function and production of reactive oxygen species
Part of American Journal of Physiology - Renal Physiology, 2018
Editorial: Hypoxia in Kidney Disease
Part of Frontiers in Physiology, 2018
- DOI for Editorial: Hypoxia in Kidney Disease
- Download full text (pdf) of Editorial: Hypoxia in Kidney Disease
Part of Mediators of Inflammation, 2017
- DOI for 15-Deoxy-Delta(12,14)-prostaglandin J(2) Exerts Antioxidant Effects While Exacerbating Inflammation in Mice Subjected to Ureteral Obstruction
- Download full text (pdf) of 15-Deoxy-Delta(12,14)-prostaglandin J(2) Exerts Antioxidant Effects While Exacerbating Inflammation in Mice Subjected to Ureteral Obstruction
Antioxidant treatment attenuates lactate production in diabetic nephropathy
Part of American Journal of Physiology - Renal Physiology, 2017
Part of Magnetic Resonance in Medicine, p. 457-461, 2017
Part of American Journal of Physiology - Renal Physiology, 2017
Hypoxia-inducible factor activation in diabetic kidney disease.
Part of Current opinion in nephrology and hypertension, p. 345-350, 2017
Metformin Normalises Medullary Hypoxia in The Diabetic Rat Kidney
Part of The FASEB Journal, 2017
Role of carbonic anhydrase in acute recovery following renal ischemia reperfusion injury
Part of The FASEB Journal, 2017
Part of International Journal of Molecular Sciences, 2017
Pronounced kidney hypoxia precedes albuminuria in type 1 diabetic mice
Part of AMERICAN JOURNAL OF PHYSIOLOGY-RENAL PHYSIOLOGY, 2016
Part of Journal of Hypertension, p. 833-835, 2016
Part of Upsala Journal of Medical Sciences, p. 12-16, 2016
- DOI for Iodinated contrast media inhibit oxygen consumption in freshly isolated proximal tubular cells from elderly humans and diabetic rats: Influence of nitric oxide.
- Download full text (pdf) of Iodinated contrast media inhibit oxygen consumption in freshly isolated proximal tubular cells from elderly humans and diabetic rats: Influence of nitric oxide.
Pancreatic islet blood flow and its measurement
Part of Upsala Journal of Medical Sciences, p. 81-95, 2016
- DOI for Pancreatic islet blood flow and its measurement
- Download full text (pdf) of Pancreatic islet blood flow and its measurement
Renal oxygenation during haemorrhage is not aggravated by angiotensin II AT1-receptor blockade
Part of Acta Physiologica, p. 153-155, 2016
Part of The FASEB Journal, 2015
Part of Diabetologia, p. 2435-2442, 2015
Part of Microvascular Research, p. 124-129, 2015
- DOI for The use of hydrogen gas clearance for blood flow measurements in single endogenous and transplanted pancreatic islets
- Download full text (pdf) of The use of hydrogen gas clearance for blood flow measurements in single endogenous and transplanted pancreatic islets
Activation of Hypoxia-Inducible Factors Prevents Diabetic Nephropathy
Part of Journal of the American Society of Nephrology, p. 328-338, 2015
Part of Acta Physiologica, p. 58-58, 2015
Part of American Journal of Physiology - Renal Physiology, 2015
Part of Acta Physiologica, p. 311-318, 2015
ET-1 increases reactive oxygen species in hypoxic glomeruli during high salt intake
Part of Acta Physiologica, p. 559-560, 2015
Part of Acta Physiologica, p. 795-804, 2015
Part of Kidney International, p. 109-115, 2015
Part of Upsala Journal of Medical Sciences, p. 233-240, 2015

