Oral drug delivery

SweDeliver Work package Oral drug delivery focus on improving the design of drug delivery systems that fulfil the requirements for absorption from the small intestine and colon.
The oral route is typically the most attractive for administration of drugs, since it is a convenient and widely accepted administration route by patients that improves compliance. However, the need for advanced oral drug delivery systems is increasing due to the physicochemical profile of contemporary drugs, making them difficult to absorb from the intestine.
This was identified by our industrial partners at the start of SweDeliver and our research programme has been developed by very strong academics Professors Per Artursson and Christel Bergström and Associate Professors Per Larsson and Alexandra Teleki in collaboration with an engaged group of industrial partners: AstraZeneca, Ferring, Janssen Pharmaceuticals, Disruptive Pharma and Orexo AB. In the last evaluation by our Scientific Advisory Board, the SweDeliver Work package Oral Drug Delivery was stated to be “top quality, reflecting outstanding work at world leading level with great international impact”.
A significant and highly appreciated part of the research conducted in this Work Package is the studies of Computational pharmaceutics. The Molecular Pharmaceutics group at Uppsala University is a pioneer within this field and currently at world leading level. Our industry partners have stressed the need to strengthen competence within this sector as we move towards a more digitalised world and technological advances enable the analysis of large-scale data sets. Therefore, more or less all experimental projects this Work Package are supported with computational pharmaceutics approaches to better understand processes of importance for intestinal absorption and make better use of the generated data.
The direct computational work conducted in this Work Package includes studies of transient permeation enhancers using molecular dynamics (MD) simulations by PhD students Rosita Kneiszl and Shahina Akter (Kneiszl et al., Mol. Pharm., 2021; Hossain et al., Nanoscale, 2023). They make use of the virtual intestine developed by Professor Bergström and colleagues, which is unique for this laboratory and not available elsewhere.

Rosita Kneiszl, SweDeliver Work Package Oral Drug Delivery
Other efforts include making use Computational Fluid Dynamics (CFD) to create an intestinal model including motility aspects (peristalsis and segmentation) to understand its role on co-localisation of excipients (permeation enhancers) and drugs (macromolecules such as insulin) after oral intake (Naranjani et al., Int. J. Biol. Macromol., 2023) (work exemplified in figure 3). This workstream was developed jointly with our former Guest Professor James Brasseur who had a 20% appointment with SweDeliver 2020-2023.
The CFD work is carried out by PhD student Benyamin Naranjani, who is currently expanding his knowledge of CFD modelling with artificial intelligence during a twinning project with AstraZeneca. Benchtop research and development of effective and physiologically predictive in vitro model remains a vital part of drug delivery. The GI tract is a highly complex environment and many factors must be considered during the development of new drug formulations.
Complementary to the MD and CFD modelling efforts, we have developed in vitro models to understand drug dissolution, solubility and diffusion in colonic mucus with the overall aim to better understand the role of the mucus barrier on absorption from colon. Artificial mucus mimicking the colonic mucus in larger preclinical species used in absorption studies (dogs and pigs) have been developed by researcher Ilse Dubbelboer and PhD students Vicky Barmpatsalou and Marco Tjakra (Barmpatsalou et al., Eur. J. Pharm. Sci., 2023; Barmpatsalou et al., Eur. J. Pharm. Sci., 2024).
As life-style diseases such as ulcerative colitis and Crohn's disease are increasing globally, local treatment of the colon is of high interest. Tjakra therefore continues this work to develop artificial mucus mimicking the colonic mucus of the healthy and diseased colon. Jointly, Tjakra and PhD student colleague Mingjun Wu are currently developing an in vitro dissolution method for studies of the importance of the mucus barrier for drug release; the physical model used for these studies is currently explored for patentability. Further, Tjakra combines this work physiology-based pharmacokinetics (PBPK) and is out for secondment to MSD in the states to develop these skills in accordance with the plans included in the EU-ETN Colotan which is the main founder for this project.
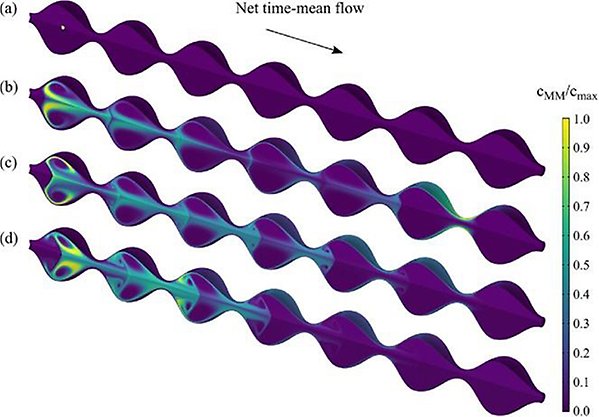
Figure 3: After oral ingestion of drugs, different contractile and mobility patterns are responsible for mixing and propelling contents along the GI tract. To better understand this phenomena Naranjanj et al have constructed an in silico model representing the peristaltic motility along the GI tract. The figure is a three-dimensional representation of the water pocket of the whole modeled segment with relevant dimensions after dosing of a drug (insulin) for varying wave speeds (0-1.5 cm s−1). ® International Journal of Biological Macromolecules, 2023.
Similar to Tjakra, PhD student Rebekkah Hammar of EU-ETN Colotan and SweDeliver has studied the influence of intra- and extracellular free drug concentrations in the human colon on local and systemic drug exposure (Hammar et al., Eur. J. Pharm. Sci., 2023). Hammar’s research has a focus on the optimisation of an organoid model of the colon, called colonoids. Hammar has managed to develop these to present the organoids in the ‘apical-out’ form in a robust manner, enabling rational studies of uptake in the colon in a small-scale format of more tissue-like cell systems than those currently in use (e.g. cell monolayers). During her secondment to AstraZeneca, Hammar has combined her skills in cell biology with studies of the microbiome to understand to what extent drugs are absorbed and affecting the microbiome naturally present in the large intestine.
Apart from the development of in vitro models, we are studying various formulations strategies – including lipid based nanoemulsions loaded with peptides. The digestion of lipids and their effect of the peptides stability and permeation have been developed by post doc Ann-Christin Jacobsen and is currently explored in a combined digestion-absorption assay by post doc Hannah Pohlit (Jacobsen et al., Drug Deliv. Transl. Res., 2022).
The generation of a generic, nano-sized drug delivery platform is being development by PhD Student Xiguo He and Associate Professor Madlen Hubert, currently focusing on engineering nanoparticles and loading liquid crystalline nanoparticles with antibiotics. Here, the effect of the antibiotics on the microbiome is also studied, with the intention to decrease adverse reactions in the gut by efficient encapsulation and increased uptake of nanoparticles by the enterocytes. The intestinal colloidal structures and their influence on self-assembly of lipid-based formulations are studied by PhD student Shahina Akter. using various screening techniques including SAXS combined with MD simulations.

Xiguo He, Madlen Hubert, Shahina Akter, SweDeliver
The Work Package have established advanced in vivo models for studies of the molecular characteristics of macromolecular drugs and to support the selection of suitable permeation enhancers to improve oral absorption. This work has been carried out in the laboratories and state-of-the art animal facility at AstraZenec. These studies were spearheaded by PhD student Staffan Berg who has established a new rat instillation model and a pig intestinal absorption model at AstraZeneca as a result of his PhD studies carried out at SweDeliver (Berg et al., Mol. Pharm., 2021; Berg et al., J. Contr. Release, 2023).
Berg focused extensively on co-localisation studies of drug and permeation enhancers whereas PhD student Prosper Emeh, continuing the work, is currently expanding the knowledge on physicochemical properties and its role in selection of permeation enhancer. Emeh also has combined in vitro and in vivo work, identifying how to select a proper model for the question asked around macromolecular intestinal absorption (Emeh et al., Mol. Pharm., 2023).
Overall, SweDeliver Work package Oral Drug Delivery has successfully expanded the research and the team with other sources of funding, including internal Uppsala University funding (Uppsala Antiobiotic Center,. He), Swedish Neutron Education for Science and Society (SWEDNESS; Akter) and EU-ETN Colotan (Tjakra, Hammar). Still, note that all Work Packages are actively engaged in applications for funding from other sources to further support the work performed.
Work package Leaders
Associate Professor Per Larsson, Department of Pharmacy, Uppsala University
Professor Bertil Abrahamsson, AstraZeneca
Doctor Krista Ojala, Orion Pharma Oy
Research projects
- Combined computational modeling at different scales to understand peptide drug and excipient co-localization
- Diffusion, dissolution, and release mechanisms for dosage forms delivering drugs to the colon: Importance of colonic mucus as a barrier to drug absorption
- Drug delivery platform for optimal intestinal absorption of oral antibiotics
- Effects of molecular characteristics of macromolecular drugs and selection of permeation enhancer on oral absorption
- Exploring the impact of the Gastrointestinal Mucus on drug absorption: Characterization and development of novel predictive tools (finished project)
- Influence of intestinal colloidal structures and self-assembly on lipid-based formulations for enhancing peptide drug bioavailability
- Influence of intra- and extracellular free drug concentrations in the human colon on local and systemic drug exposure
- Investigation of oral absorption of macromolecules using in silico techniques
- Investigation of The Interplay Between Permeation Enhancers, Gi Physiology and Macromolecular Drugs to Facilitate Design of Oral Dosage Forms (finished project)
- In vivo dissolution in colon of poorly soluble drug substances
- Local intestinal generation of transient permeability enhancers by digestion of lipid formulations (finished project)
- Mechanistic modeling of peptide drug permeability
