Parenteral Drug Delivery

SweDeliver Work package Parenteral drug delivery focus on the development of new formulations and improved delivery systems for subcutaneous drug administration.
Parenteral drug delivery is the most important administration route, always needed to ensure that the drug can be administered to e.g. non-conscious patients, while also being a critical administration route to better understand toxicity and bioavailability.
Additionally, a growing number of novel molecules in the drug discovery pipeline are biological compounds like proteins, peptides, and RNA which are primarily administered parenterally. Hence, this administration route has received a renewed interest from the pharmaceutical industry.
Traditionally, the research performed at Uppsala University has been carried out in disparate research projects at the faculty without joint strategies and little synergistic effects. The SweDeliver industrial partner group identified the need for a strategy around parenteral drug delivery to better respond to industrial needs and to safeguard that competence within this area was continued to be built at the European level.
Already two years in, the SweDeliver Scientific Advisory Board commented that the competence center's Work Package Parenteral Drug Delivery – through very active engagement from academic researchers Professors Per Hansson and Sara Mangsbo and Associate Professors Magnus Bergström and Erik Sjögren (shared employment between Uppsala University and Pharmetheus) and industrial partners (key researchers from Affibody AB, AstraZeneca, CTC AB, DelSiTech, Emplicure AB, Ferring, Orion Pharma, Ultimovacs AB and Janssen Pharmaceutia) – have reached an international high standard (excellent work, next to world-leading level) with the potential to reach the next level.
In this part of our research programme, large emphasis has been put on various aspects of subcutaneous administration. Here, method development to predict the behaviour of pharmaceuticals in subcutaneous tissue has been extensively investigated. A sophisticated in vitro model to study the interaction between drugs and hyaluronic acid based on microfluidics have been developed (Figure 1, below) and a patent has been filed by Marcus Wanselius and PI Per Hansson (Microfluidics platform for studies of peptide – polyelectrolyte interaction, Wanselius et al., Int. J. Pharm, 2022).
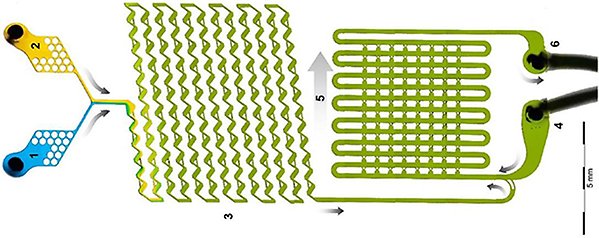
Figure 1: Picture of the microfluidic chip developed by Wanselius et al for studying the interaction between the drug and hyaluronic acid. The novel in vitro model will contribute to deeper understanding of the behaviour of pharmaceuticals in subcutaneous tissue. 1: inlet for solvent, 2: inlet for stock solution containing the drug molecule, 3: mixer for solvent and drug solution, 4: inlet for microgels, 5: microgel traps and 6: oulet for liquid. ®International Journal of Pharmaceutics, 2022.
To better understand the IP field and potential of the idea, Wanselius partook in courses and training at Uppsala University Innovation, including UUI Mentor Programme and Uppsala Innovation Center (UIC) business startup, a business development program for researchers. The method is currently used by PhD student Anton Norberg to investigate how the presence of albumin affects the interaction of drug peptides with polyelectrolyte networks.
To better understand the diffusivities of peptide drugs in the extracellular matrix, an in vitro model based on self-gelling extracellular matrix model (ECMM) consisting of collagen I, hyaluronic acid, and chondroitin sulfate has been developed by post docs Agnes Rodler, Yassir Al-Tikriti and David Juriga. Rodler further developed a tool for analysis of Fluorescence Recovery After Photobleaching (FRAP) experiments in rectangular geometry, which provided more accurate determinations of diffusion coefficients compared with the simple circular bleaching methods. Small-angle X-ray scattering (SAXS) investigations of the microstructure of ECMM was also performed in order to determine mesh size and collagen nanostructure.

Agnes Rodler, Yassir Al-Tikriti and David Juriga, SweDeliver
A method based on FRAP is also used by PhD student Julia Parlow to study the interactions between the peptides and the matrix. To ease the analysis of the data, a user-friendly computer program for rapid data processing and model analysis of the generated FRAP data was developed by researcher Jonas Gernarndt. Gernarndt established various models to describe diffusion under different conditions, which are now being implemented in other WP1 research projects with potential use also in the WP2 projects with a focus on drug diffusion in mucus. These models will be packaged as a computational tool, provided free of charge to the public. In addition, the models are now being transferred to industrial partners with an interest in analysis of any FRAP generated data.
Another way of studying subcutaneous drug delivery is by the means of in silico tools. In WP1, a subcutaneous physiological based biopharmaceutics model has been developed by post doc Ilse Dubbelboer and PI Erik Sjögren following an extensive review of scientific literature (Dubbelboer & Sjögren, Int. J. Pharm, 2022; Dubbelboer & Sjögren, Eur. J. Pharm. Sci, 2022). The model is currently being evaluated with often used preclinical species and small molecules, partly based on input from our industrial partners and partly based on high quality literature data.
The models are close to completion: data has been collated and checked, and models have been created in the original PK-Sim software. This is a freeware, making the models possible to transfer to any laboratory inside or outside of SweDeliver, a decision made to enable the models to be utilised without the need of expensive licenses. A second reason for SweDeliver to choose freeware is to certify that we do not become biased to particular commercial software.
Selection of the right drug molecules for optimal treatment are of course also of vital importance. In the project Immunogenicity of synthetic long peptides and the role of formulation and structure for efficacy and toxicity lead by post doc Martin Lord, immunogenic profiling and structural profiling of the Sars-CoV-2 derived peptides have been investigated (Eriksson et al., Chembiochem., 2023). Here, Lord synthesized a peptide, SARS-10, with a short static peptide stretch called the pTag that can conjugate the peptide to specially designed antibody carriers. Metal-organic frameworks (MOF) was identified as alternative nanocarriers of peptides. A structural investigation of the MOFs and antibodies were conducted with SAXS in collaboration with Per Hansson and cellular uptake and tracking studies were performed. In collaboration with Julia Parlow, FRAP-studies of the bispecific antibody formulation in extracellular matrix hydrogels have been performed.
Work package Leaders
Professor Per Hansson, Department of Medicinal Chemistry, Uppsala University
Doctor Susanna Abrahmsén Alami, AstraZeneca
Research projects
- Amphiphilic Drugs in Microgels (Completed project)
- Amphiphilic Properties of Drug Molecules and Their Self-Assembly in Presence of Phospholipids
- Immunogenicity of synthetic long peptides and the role of formulation and structure for efficacy and toxicity (Completed project)
- Investigation of the relationship between the in vitro properties of different subcutaneously administered peptides and their bioavailability and absorption in vivo
- In Vitro Methods for Enhanced Understanding of Peptide Transport in Subcutaneous Tissue
- Novel in vitro methods to understand transport properties of biologics (Completed project)
- Novel In Vitro Models for Subcutaneous Administration of Drugs: Transport Properties
- Novel In Vitro Models for Subcutaneous Administration of Drugs (Completed project)
- Physiochemical Aspects of Subcutaneous Administration of Drugs (Completed project)
- Physiologically based biopharmaceutics modeling for subcutaneous drug administration
- Self-assembly of therapeutic peptides
- Subcutaneous administration of biotherapeutics: In vitro properties and bioavailability and absorption rate in vivo
