Klebsiella pneumoniae biofilms: from virulence to antibiotic resistance
Bacterial biofilms are aggregates of cells, attached to a surface or not, that are enclosed in an extracellular matrix (ECM) that consists of extracellular polymeric substances (EPS) produced by the bacteria. These can include polysaccharides, proteins, and nucleic acids, as well as lipids, amyloids, cellulose, fimbriae, pili, flagellae and cell debris. The proximity between cells in the biofilm facilitates the establishment of physical and social interactions that benefit the population and the ECM, together with different growth stages of cells in different parts of the biofilm, protects bacteria from exposure to antibacterial agents and the host immune system. This results in more complicated infections, need for longer treatments and increased risk of treatment failure. Besides, temporally increased antibiotic tolerance in biofilms could potentially represent an opportunity for the development of genetically determined resistance.
Since the protective effect against antibiotics in biofilms differs between different antibiotics and different bacterial species, it is important to increase understanding of the underlying mechanisms of biofilm development and properties in order to improve treatment procedures. Recent studies show that there is a selection of bacterial populations with an altered biofilm capacity during infection, rapidly creating subpopulations with different virulence and potentially different antibiotic susceptibility. How such changes in biofilm capacity affect infection progression and treatment of biofilms are largely unstudied. To design better therapeutics for patients with infections involving biofilm growth, it is vital to understand dynamics of selection in biofilms and how bacteria with altered biofilm capacity respond to antibiotics.
In these projects, we are using Klebsiella pneumoniae, a nosocomial pathogen that poses an increasing threat due to biofilm formation and transmission of multiresistance plasmids, as a model for the study of biofilm dynamics and susceptibility to antibiotics of hypervirulent K. pneumoniae strains that have an altered biofilm capacity.
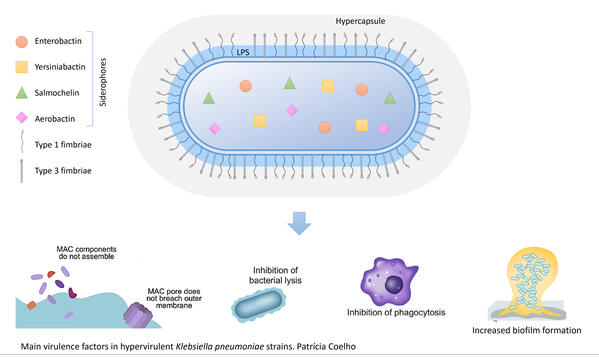
Project I: How does altered biofilm capability affect antibiotic efficacy?
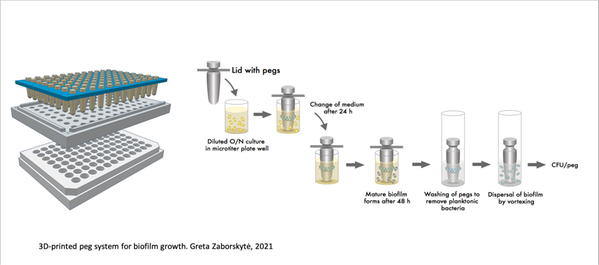
In this project, we will study how newly identified mutations affecting K. pneumoniae capsule and biofilm formation impact bacterial susceptibility to antibiotics using an in-house developed system based on bacterial growth on separate 3D-printed pegs that are particularly well suited for evolution and analysis of treatments of biofilms. We will evaluate how the observed mutations affecting capsule production and biofilm formation interfere with bacterial susceptibility to clinically relevant antibiotics.
Project II: Is selection of resistance and the spread of resistance plasmids affected by biofilm growth?
In biofilms, bacteria at different positions will experience different antibiotic concentrations, which may have important effects on selection and emergence of resistant bacteria. To address whether resistance mechanisms selected in biofilms differ from those prevalent in planktonic cultures, we will use mixed biofilms consisting of differentially fluorescently labelled resistant and susceptible K. pneumoniae in the presence and absence of different concentrations of antibiotics to determine the lowest drug concentration that results in enrichment of the resistant strain.
Moreover, biofilm growth is often proposed to enhance plasmid spread by conjugation, since it is believed to promote direct cell-cell contact. However, extracellular matrix and the lack of cell mobility may restrict new contacts and alter the conjugation dynamics. We aim to measure the rate of conjugation as a function of antibiotic concentration at different stages of biofilm growth at the population level and also visualize it at different positions in the biofilm.
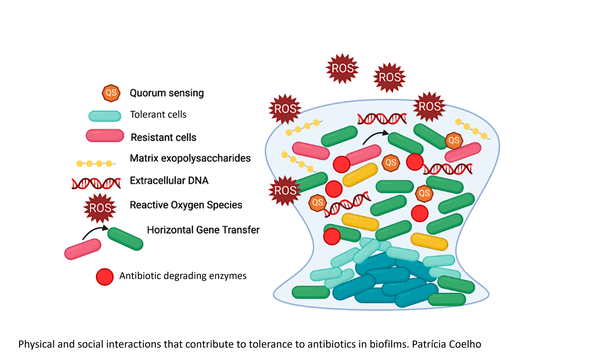
Project III: Can antibiotic combination therapy improve treatment of biofilms?
From the results on the antibiotic susceptibility of biofilm mutants, effects of antibiotics on biofilms, and evolution of resistance in the projects described above, we will design and test novel treatment combinations for further improvement of treatment and prevention of selection of mutants in biofilms, using combinations of β-lactams and novel β-lactamase inhibitors to test if treatment efficiency of biofilms can be improved.
Related published research
- Erik Gullberg ,Sha Cao, Otto G. Berg, Carolina Ilbäck, Linus Sandegren, Diarmaid Hughes & Dan I. Andersson. 2011. "Selection of Resistant Bacteria at Very Low Antibiotic Concentrations", Jul;7(7):e1002158.
- Erik Gullberg, Lisa M. Albrecht, Christoffer Karlsson, Linus Sandegren & Dan I. Andersson. 2014. “Selection of a Multidrug Resistance Plasmid by Sublethal Levels of Antibiotics and Heavy Metals”. mBio, Oct 7;5(5):e01918-14.
- Jonas Stenløkke Madsen, Mette Burmølle, Lars Hestbjerg Hansen & Søren Johannes Sørensen. 2012. “The interconnection between biofilm formation and horizontal gene transfer”. FEMS Immunology and Medical Microbiology, Jul;65(2):183-95.
- Michelle Paczosa & Joan Mecsas. 2016. “Klebsiella pneumoniae: Going on the Offense with a Strong Defense”. Microbiology and Molecular Biology Reviews, Jun 15;80(3):629-61.
- Thomas Russo & Candace M. Marr. 2019. "Hypervirulent Klebsiella pneumoniae". American Society for Microbiology, May 15;32(3):e00001-19.
- Thibault Stalder & Eva Top. 2016. “Plasmid transfer in biofilms: a perspective on limitations and opportunities”. NPJ Biofilms Microbiomes, 2:16022.
- Chloé Virolle, Kelly Goldlust, Sarah Djermoun, Sarah Bigot and Christian Lesterlin. 2020. “Plasmid Transfer by Conjugation in Gram-Negative Bacteria: From the Cellular to the Community Level”. Genes, Oct 22;11(11):1239.
- Zaborskytė, Greta. 2021. “Modular 3D printed peg biofilm device for flexible setup of surface-related biofilm studies”. Frontiers in Cellular and Infection Microbiology (pending final publication)
