A generic nanosized drug delivery platform for optimal absorption of antibiotics
Global antibiotic consumption has increased significantly over the last two decades due to improved access to health care. This trend is expected to continue. Oral therapy not only circumvents medical complications associated with intravenous applications, but is also less expensive and can shorten hospital stays. However, most antibiotic drugs suffer from low stability in the gastrointestinal tract (GIT), poor water solubility, as well as low permeability across biological membranes, resulting in limited absorption across the intestinal epithelial barrier. The high antibiotic doses required for adequate efficacy result in a large fraction of the dose not being absorbed from the intestine. The dose left in the intestine both changes the composition of the gut microbiota and can promote the development of bacterial resistance.
Formulating drugs into nanocarriers is one strategy to increase drug absorption. In addition, nanocarriers also enhance solubility and chemical stability. In this project, more efficient drug delivery systems are designed that specifically deliver the drug to the site of absorption in the GIT. We will design interdisciplinary nanoparticles that can solubilize and transport an effective drug dosage to the intestinal epithelium for complete absorption. Focus is set on the nature of the building blocks to design nanocarriers that are stable in the GIT but at the same time highly membrane-permeable. Nanotechnology will be coupled to in vivo relevant digestion-permeation assays to determine the biological fate. Furthermore, mechanistic cell studies will allow the exploration of molecular interactions and mechanisms behind absorption.
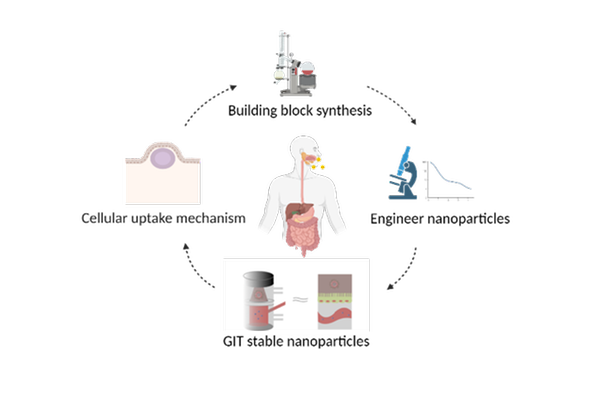
Scheme of the process of nanoparticles development
Related published research
- Janneke Keemink, Elin Mårtensson, and Christel A.S. Bergström, “Lipolysis-Permeation Setup for Simultaneous Study of Digestion and Absorption in Vitro” Molecular Pharmaceutics 16, no. 3 (2019): 921–30,
- Oliver J. Hedge and Christel A. S. Bergström, “Suitability of Artificial Membranes in Lipolysis-Permeation Assays of Oral Lipid-Based Formulations,” Pharmaceutical Research 37, no. 6 (May 20, 2020): 99
- Letícia Rodrigues, Fabian Schneider, Madlen Hubert et al., “Cellular Uptake of Self-Assembled Phytantriol-Based Hexosomes Is Independent of Major Endocytic Machineries,” Journal of Colloid and Interface Science 553 (October 2019): 820–33
- Leah Wright, Paul Joyce, Christel A.S.Bergström, Madlen Hubert et al., “A Comparison of Chitosan, Mesoporous Silica and Poly(Lactic-Co-Glycolic) Acid Nanocarriers for Optimising Intestinal Uptake of Oral Protein Therapeutics,” Journal of Pharmaceutical Sciences 110, no. 1 (January 2021): 217–27
- Janneke Keemink, Oliver J.Hedge, Valentina Bianco, Madlen Hubert & Christel A.S. Bergström, “Comparison of Cellular Monolayers and an Artificial Membrane as Absorptive Membranes in the in Vitro Lipolysis-Permeation Assay,” Journal of Pharmaceutical Sciences 111, no. 1 (2022): 175–84.
