Research projects in Widersten Group
Our modern society would be unthinkable without the constant development and production of new chemicals. These are indispensable building blocks in our materials, pharmaceuticals, as fuel for our vehicles and in research and development. Production of chemicals has traditionally been, and still is, almost entirely dependent on the availability of oil-based raw materials. In addition, rare transition metals are often used as catalysts in the synthesis and large amounts of waste are created, which in many cases require special handling due to its toxicity.
An important step towards more environmentally friendly production of necessary chemicals would be to change the synthesis pathways towards long-term sustainable technologies. Nature's own catalysts, enzymes, have long been highly attractive for use in chemical production as they would bring several advantages over traditional synthesis methods. The amount of hazardous waste could be drastically reduced as enzymes are biodegradable, work in aqueous solution and eliminate the need for rare transition metals. The same applies to energy consumption because enzymes work efficiently at room temperature, unlike traditional catalysts that often require heating of the reaction mixture. In addition, enzymes are characterised by an unprecedented efficiency and selectivity. Often, enzymes can distinguish different stereoisomers of the same molecule and accept only one isomer as a substrate, making them very attractive as catalysts for chiral synthesis where product purity is crucial.
In our research, we study a variety of enzymes in order to understand which properties make them effective chemical catalysts. This mapping is important as the new knowledge makes it possible to develop completely new enzyme variants with specially adapted properties. New enzyme variants are produced through a process called directed evolution. Genes encoding the enzymes are altered in a controlled process that results in new enzyme variants that carry various changes in their active sites where the chemical reaction takes place and is catalysed. In the process, a variety of enzyme variants are formed, all with more or less altered properties. From these libraries of altered enzymes, we select the variants that exhibit properties in the direction of the desired ones, i.e. improved versions of the starting enzyme that for example could be used for large-scale production of industrially relevant chemicals. One of our goals is to construct multi-step reactions where all reaction steps are enzyme-catalysed.
Epoxide hydrolases
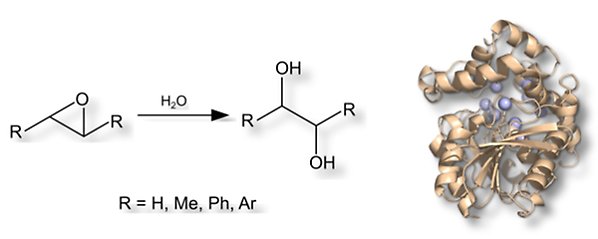
The epoxide hydrolase StEH1 from potatoes has proven to be a very promising enzyme catalyst for the hydrolysis of epoxides. The enzyme is stereoselective, easy to produce, structurally stable even after mutagenesis and we have collected a lot of structure/activity data in recent years. We study this enzyme and laboratory-developed enzyme variants through a combination of function and structure analyses as well as computational chemical methods. The goal is to isolate new stereoselective enzymes that are suitable as biocatalysts.
Alcohol dehydrogenases
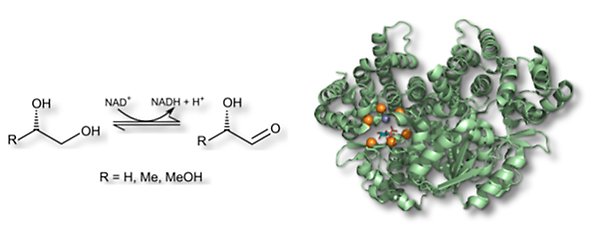
Propanediol oxidoreductase (FucO), from Escherichia coli, is a diol dehydrogenase that catalyses the oxidation of short aliphatic alcohols such as 1,2-propanediol to the corresponding aldehyde. The enzyme is regioselective (reacting only with primary alcohols) and enantioselective. FucO belongs to group III of alcohol dehydrogenases which depend on an iron(III) ion for their activity. The structure of the enzyme is adapted to allow binding of low-molecular compounds such as the physiological substrate S-1,2-propanediol. Through mutagenesis and directed evolution, we want to expand substrate selectivity to include larger alcohols, such as aromatic diols.
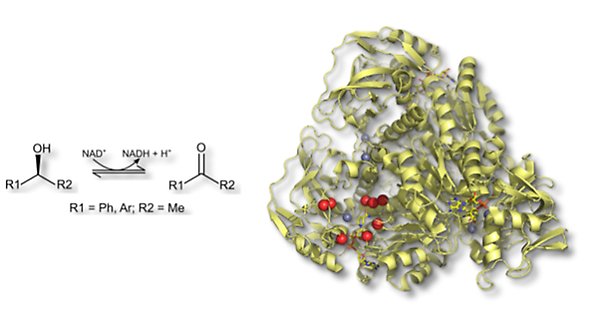
The alcohol dehydrogenase from Rhodococcus ruber DSM 44541, ADH-A, catalyses the oxidation of secondary alcohols to the corresponding ketones. ADH-A is a NAD+/NADH and zinc-dependent alcohol dehydrogenase, whose structure is closely related to previously well-characterized isoenzymes from horse liver and yeast. ADH-A shows good activity with phenyl-substituted secondary alcohols but relatively low activity with vicinal diols. The low diol activity makes it interesting to isolate ADH-A variants that show good activity even with aryl-substituted diols through mutagenesis and directed evolution.
Aldolases
Adol reactions are a cornerstone of organic synthesis as they enable the formation of new carbon-carbon bonds while forming new stereocenters. Aldol reactions are utilised in the synthesis of a variety of hydroxylated compounds. These are of particular interest in the synthesis of bioactive substances and can be formed from α-hydroxylated ketones and aldehydes. We study 2'-deoxy-5-phosphate-ribosaldolase (DERA) and fructose-6-phosphate aldolase (FSA) from Escherichia coli with a focus on increased understanding of kinetic mechanisms and evolution of new substrate preferences. The goal is to broaden substrate selectivity to also include non-phosphorylated substrates, such as aryl-substituted aldehydes.
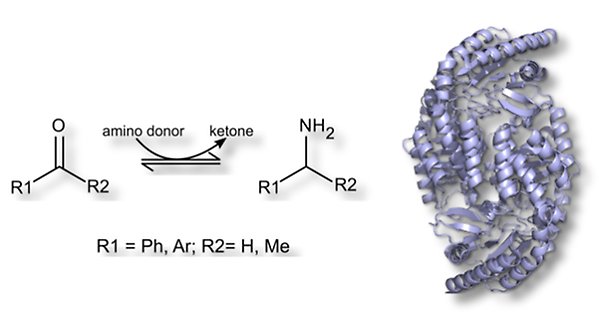
Aminotransferases
Amine:pyruvate aminotransferase from Vibrio fluvialis JS17, APA, catalyses PLP-dependent transfer of amino groups from amines and amino acids to α-keto acids, aldehydes and ketones. APA exhibits some activity with phenyl-substitute substrates, however lower with larger amine acceptors. This makes it interesting to study APA's activity with ketones with different substituents and to introduce mutations to increase APA's activity with larger acceptors as well as with α-hydroxyketones (acyloins). The goal is to isolate novel APA variants that stereoselectively catalyse amino group transfer to aryl substituted and hydroxylated aminoacceptors.