Andor Pivarsci research group
%20grupp.webp)
Description of the research
Non-coding RNAs (ncRNAs) are transcripts that do not primarily function by encoding for proteins, but instead play regulatory roles in our cells. Long non-coding RNAs (lncRNA) and microRNAs (miRNAs) have emerged as fundamental regulators of cellular processes and critical players in diseases.
We have been studying the role of non-coding RNAs in the regulation of skin homeostasis, development and diseases such as skin cancer and inflammation. In particular, we aim to understand how non-coding RNAs contribute the development and progression of keratinocyte-derived cancers, the functional decline of the skin during ageing, as well as the development of chronic inflammatory skin diseases. With the recent development in RNA-based medicine, knowledge and understanding of non-coding RNA gene networks in development and diseases has the potential to transform clinical practices and lead to improved treatment modalities for patients with cancer, inflammatory and genetic diseases.
Projects
Non-Coding RNAs in Skin Stem Cell Differentiation and Keratinocyte-Cancers
PI: Professor Andor Pivarcsi
Our overarching aim is to understand the role of non-coding RNAs, important regulators of cell differentiation and organ development, in the pathogenesis of the disease (Figure 3). Via the systematic molecular analysis of patient-derived samples from tumours and precancerous lesions, and in vitro and in vivo cancer models, we aim to understand their role in the development and progression of the disease. Our main research focus currently is the understanding of the role of long non-coding RNAs (lncRNAs) in one of the most common human cancers, squamous cell carcinoma of the skin (cSCC). Our results have identified important roles for ncRNAs in skin development, tissue differentiation and ageing, in the emergence and progression of cancer. We have also identified a set of highly skin-specific non-coding RNAs, whose functions are unknown and may hold the key to the understanding of skin development.
The group uses patient material to identify novel disease-relevant non-coding RNAs by transcriptomic analyses. Moreover, CRISPR-Cas9-based loss-of-function and gain-of-function experiments in cell culture models, 3D reconstructed tissue equivalents and mouse disease models are used to identify the exact molecular role of disease-relevant ncRNAs, as well as, to an increasing extent, data analysis with bioinformatics methods.
%20Fig.2%20ncRNAs%20in%20skin%20ageing%20and%20cancer.webp)
%20Fig.4.%20LINC_DAPI_cSCC_RNAScope.webp)
One of the cSCC-associated lncRNAs we identified is Plasmacytoma Variant Translocation 1 (PVT1). Investigation of PVT1 transcript isoforms revealed a conserved exon shared among multiple PVT1 isoforms, exon 2, in the PVT1 transcript. Structural analysis of the PTVT1-transcript revealed that this sequence forms a distinct structure. Interestingly, the knockout of PVT1 exon 2 impaired cSCC tumour growth both in vivo and in vitro assays demonstrating that exon 2 is a critical element for the oncogenic role of PVT1.
%20Fig.3.%20ncRNA_SCC_workflow.webp)
Visualization of skin cancer (cSCC) -associated long non-coding RNAs by single molecular RNA in situ hybridization. Blue signal denotes cell nuclei, yellow signal represents a long intergenic non-coding RNA overexpressed in cutaneous squamous cell carcinoma tissue.
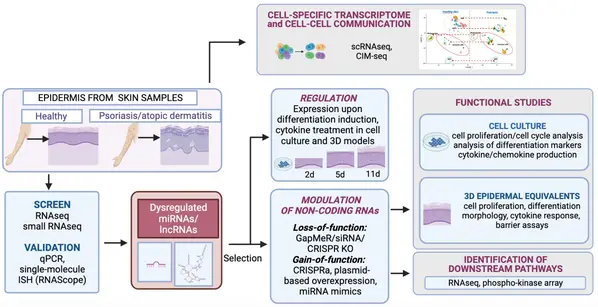
Non-Coding RNAs in Chronic Inflammatory Skin Diseases: Psoriasis and Atopic Dermatitis
PI: Professor Enikö Sonkoly
Psoriasis and atopic dermatitis are the most common chronic inflammatory skin diseases. The Sonkoly-group is studying the molecular background of these diseases, and showed for the first time in 2007 the involvement of miRNAs, short non-coding regulatory RNAs, in psoriasis and atopic dermatitis. Subsequent studies from the group have demonstrated important regulatory roles for specific miRNAs as well as long non-coding RNAs in inflammatory responses in the skin and regulating the balance of epidermal proliferation/differentiation. The group uses a combination of patient samples, animal models, cell culture and 3D epidermal models, to address the following research questions:
- Which regulatory non-coding RNAs are dysregulated in chronic inflammatory skin diseases and in which cells?
- Which biologic processes, genes and signaling pathways are regulated by the disease-associated non-coding RNAs in the skin? What is the biological function of the disease-associated non-coding RNAs? What is their mechanism of action?
- Can therapeutic modulation of non-coding RNAs be beneficial in chronic inflammatory skin diseases?
In addition, current investigations aim to create an atlas of the cell-specific coding and non-coding RNA signature in psoriasis epidermis, and identify cells that interact closely with each other within the epidermal compartment in psoriasis skin lesions.
Skin barrier and genetic skin diseases
PI: Enikö Sonkoly and Andor Pivarcsi
We are investigating the genetic and epigenetic regulation of epidermal development and the skin barrier. A functional skin barrier is essential to protect us from water loss, infections, chemicals and other environmental factors. Genetic disorders such as ichthyosis and other inherited skin diseases are characterized by impaired skin barrier function have a substantial negative effect on the affected individuals and their families’ lives. We investigate the molecular events during skin barrier formation in 3D skin models (Figure 2 showing successive formation of the skin barrier in this model) and the pathogenesis of genetic skin diseases (genodermatoses) including the role and therapeutic potential of regulatory RNAs in the process in a translational setting as a collaboration between the university hospital and academic research labs using patient-derived cells and lab-grown skin models.
The group is part of an EU COST action network (NETSKINMODELS; European Network for Skin Engineering and Modeling, A21108, netskinmodels.com), driving the development of cell-based skin models for dermatological research.
%20pivarsci.webp)
Development of three-dimensional (3D) epidermal equivalents. By culturing primary keratinocytes at an interphase of liquid growth medium and humified air over a period of 11 days, three-dimensional epidermis like structures can be developed, similar to that in the human skin. The expression and proper localization of key players of epidermal differentiation such as keratin 10 (KRT10), involcurin (IVL), filaggrin (FLG) can be visualized using immunofluorescence analysis. Cell membranes and cell nuclei are stained with green and blue colours, respectively
The group is part of an EU COST action network (NETSKINMODELS; European Network for Skin Engineering and Modeling, A21108, netskinmodels.com), driving the development of cell-based skin models for dermatological research.
