Lena Kjellén research group
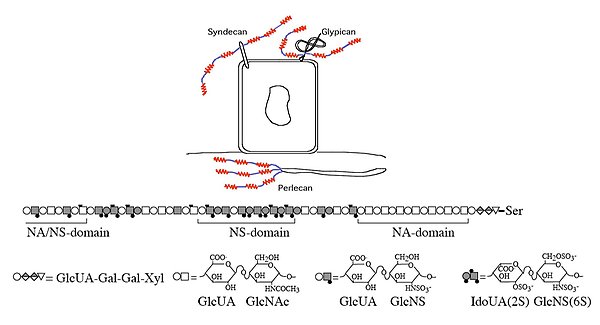
The cell surface proteoglycans syndecan and glypican and matrix proteoglycan perlecan all contain heparan sulfate chains with highly sulfated regions (“red” NS-domains), non-sulfated domains (“blue”NA-domains) and domains with intermediate sulfation (NA/NS-domains).
Heparan sulfate biosynthesis and function
Heparan sulfate proteoglycans are present on all (or nearly all) cells in the human body. They are also present in basement membranes and in the extracellular matrix surrounding many cells. The negatively charged heparan sulfate carbohydrate chains are decorated with sulfate groups creating unique cell-specific patterns. These are recognized by interacting proteins (growth factors, cytokines, morphogens, enzymes, extracellular matrix proteins etc.) where the various proteins often prefer different patterns for binding. Interactions between heparan sulfate and proteins are known to affect several physiological as well as pathological processes including embryonic development, cell adhesion, angiogenesis, cancer and neurodegeneration.
Our research is aimed at understanding how the cell biosynthesis machinery is regulated to design heparan sulfate sulfation patterns. Biosynthesis occurs in the Golgi compartment of the cell where several enzymes are needed to build the heparan sulfate chain and create the sulfation patterns. Current work in this area includes characterization of enzyme complexes and newly discovered biosynthesis intermediates. In addition, we are in collaboration with geneticists characterizing pathological mutations of biosynthesis enzymes.
We are also interested in degradation of heparan sulfate and have generated zebrafish models to find ways to treat a group of rare autosomal recessive lysosomal storage diseases, Sanfilippo syndrome A, B and C, which cause fatal brain damage. These patients have a reduced ability to degrade heparan sulfate which instead accumulates and causes progressive damage to neurons as well as other cells.
