Carl-Johan Rubin research group
Genetics of diseases, size, body morphology and pigmentation in horse
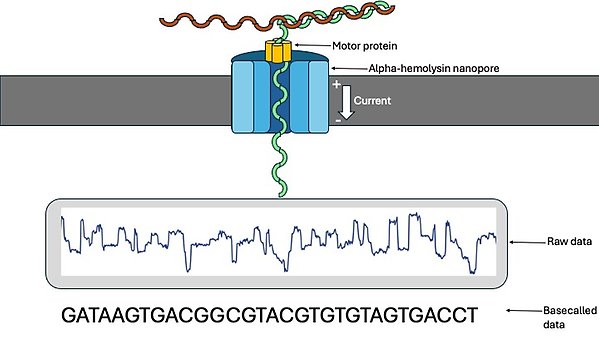
The research program centers around deciphering the impact of genetic- and epigenetic variation on sex-determination (SD), organismal growth, behavior and ageing. Most work is performed using vertebrate model species and methods applied include various DNA and RNA-sequencing applications, including Oxford nanopore sequencing, statistical genetics, cell culture and precision cas9 editing.
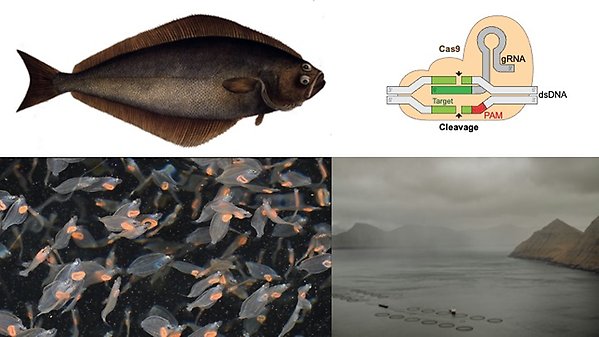
Fish have a high rate of turnover of SD systems, e.g. X/Y or W/Z chromosomes. In Atlantic halibut we previously mapped the SD chrY mutation, a lowly methylated transposon acting as a promoter for ectopic expression of the gene gsdf in males in early embryogenesis. We are now mapping loci governing sex determination in several other fish species, following up with in-vivo gene editing in flatfish to determine minimum viable makeups sex chromosomes.
We recently showed that, flatfish display heterochiasmy (HC), i.e. sexes differ greatly in meiotic recombination crossover locations and aim to describe the molecular mechanisms behind HC and to study its connection with sex chromosome turnover. Results from these model systems may lend themselves to innovation related to human medicine, such as infertility and precision editing.

Other ongoing projects involve genetics of body weight and metabolism in chicken and effects of stress on DNA methylation and behavior in salmon. In the former project we employ statistical genetics on WGS data from >3000 chicken to identify alleles affecting growth. In the latter project we investigate genome-wide effects of external stressors on DNA methylation across tissues as well as behavior. Loci responding epigenetically to stress may be targeted to alleviate deleterious effects of stress in human as well as to promote salmon welfare in aquaculture settings.
Recent method development advances include those aimed at (1) deciphering epigenetic states and (2) single cell genomics. A project in collaboration with the hospital is aimed at advancing our understanding of molecular pathways governing the “the methylation clock” (mClock), the phenomenon that DNA methylation profiles at <100 loci can predict the age of the DNA donor. The mClock is predictive of near-to-intermediate term disease and death, which makes mClock loci promising targets for improving human lifespan/healthspan. Patient material and primary cells cultures will be used to expose and modulate the inner workings of the mClock.
*Photograph/image credits to Maria Teneva on Unsplash, The Institute of Marine Research and MidJourney